Abstract
The crystal structure of the title compound, C16H28O, features C—H⋯O hydrogen bonds making C(6) zigzag chains along one 21 screw axis. Within the limits of the data collection affected by crystal quality, the Hooft parameter gave correct indications of the known molecular chirality based on the single O atom anomalous dispersion in contrast to the indeterminate Flack value. Synthetic steps starting from manool are reported.
Related literature
For details of the synthesis, see: Evans & Grant (1997 ▶); Grant et al. (1988 ▶); Vlad et al. (1978 ▶, 1983 ▶). For the related structure methyl 8,9-epoxy-12-oxo-13-oxototarane-14β-carboxylate, see: Cambie et al. (1988 ▶). For a description of the Cambridge Structural Database, see: Allen (2002 ▶). For hydrogen-bond motifs, see: Bernstein et al. (1995 ▶). For determination of absolute configuration, see: Hooft et al. (2008 ▶).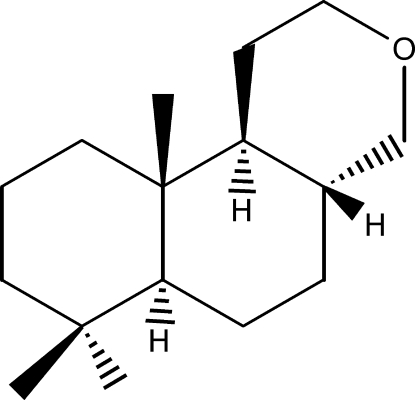
Experimental
Crystal data
C16H28O
M r = 236.38
Orthorhombic,
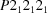
a = 7.3497 (2) Å
b = 11.1642 (3) Å
c = 17.0758 (12) Å
V = 1401.13 (11) Å3
Z = 4
Cu Kα radiation
μ = 0.50 mm−1
T = 123 K
0.70 × 0.40 × 0.13 mm
Data collection
Rigaku Spider diffractometer
Absorption correction: multi-scan (ABSCOR; Higashi, 1995 ▶) T min = 0.687, T max = 1.0
5141 measured reflections
1969 independent reflections
1823 reflections with I > 2σ(I)
R int = 0.033
θmax = 58.9°
Refinement
R[F 2 > 2σ(F 2)] = 0.034
wR(F 2) = 0.091
S = 1.06
1969 reflections
158 parameters
H-atom parameters constrained
Δρmax = 0.14 e Å−3
Δρmin = −0.12 e Å−3
Absolute structure: Flack (1983 ▶), 801 Friedel pairs
Flack parameter: −0.4 (4)
Data collection: CrystalClear (Rigaku, 2005 ▶); cell refinement: FSProcess in PROCESS-AUTO (Rigaku, 1998 ▶); data reduction: FSProcess in PROCESS-AUTO; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP in WinGX (Farrugia, 1999 ▶) and Mercury (Macrae et al., 2008 ▶); software used to prepare material for publication: SHELXL97 and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811038694/zj2024sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811038694/zj2024Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811038694/zj2024Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C6—H6A⋯O1i | 0.99 | 2.54 | 3.474 (2) | 158 |
Symmetry code: (i) 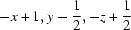 .
.
Acknowledgments
We thank the MacDiarmid Institute for Advanced Materials and Nanotechnology for funding of the diffractometer equipment.
supplementary crystallographic information
Comment
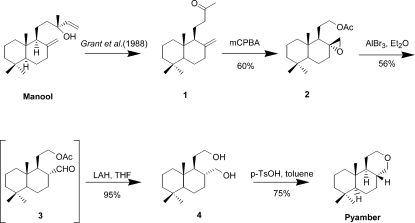
The title compound ("pyamber") was synthesized from methyl ketone 1, which is readily synthesized from manool in multi-gram quantites (Grant et al., 1988), in 4 synthetic steps (Fig. 1). A Baeyer-Villager insertion reaction of neat 1 was achieved using mCPBA to afford the previously described acetate 2 in good yield (Evans & Grant, 1997). Treatment of 2 with aluminium bromide in anhydrous diethyl ether gave one major product, aldehyde 3, which was not characterized but then immediately reduced with lithium aluminium hydride to afford diol 4 in moderate yield for the two steps (Vlad et al., 1978). Cyclization through dehydration was readily achieved under Dean and Stark conditions to afford crystalline pyamber in good yield, following chromatography; the analytical data was identical to that previously reported by Vlad et al. (1983).
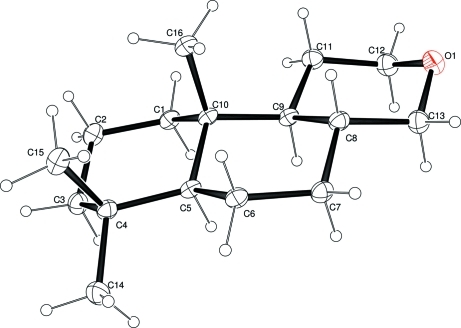
The title compound, C16H28O, crystallizes with one independent molecule in the asymmetric unit (Fig. 2). Only confirmation of structure was required for this study, with the absolute configurations of C5(S), C8(R), C9(S) & C10(S) expected from the synthesis. The Flack parameter is hardly convincing, though the Hooft equivalent parameter (Hooft et al., 2008) at -0.13 (17), with clear outliers removed [PLATON (Spek, 2009)], provides a probability of being false (P3) of 0.6 x 10-9 [P3(true) & P3(rac-twin) were 0.998,0.002 respectively]. The crystal quality/data collection also was not optimum (see experimental), but even including all measured data (2547 reflections) the Hooft equivalent parameter is -0.15 (17) while the Flack parameter changes to 0.2 (8).
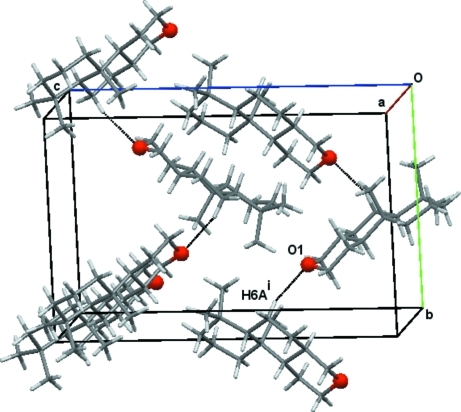
There are very few structures reported with these same three fused six-membered rings (Allen, 2002. CSD version 5.32, with May 2011 update); each ring is in a standard chair conformation. The closest related compound is the epoxide structure methyl 8,9-epoxy-12-oxo-13-oxototarane-14β-carboxylate (Cambie et al., 1988) reported in the inverted configuration. The molecules pack in zigzag chains along the b 21 screw axis bound by one C—H···O hydrogen bond (Fig. 3, Table 1) described by the motif C(6) (Bernstein et al., 1995). These chains are efficiently interlocked in the other two cell directiions via van der Waals interactions.
Experimental
2-[(2S,4aS,8aS)-5,5,8a-Trimethyl-octahydro-1H-spiro[naphthalene -2,2'-oxirane]-1-yl]ethyl acetate (2): mCPBA (26.4 g, 50% b.w., 76.4 mmol) was added portionwise to a stirred solution of methyl ketone 1 (5 g, 19.1 mmol) in chloroform (150 ml). The round bottom flask was transferred to a rotavapor and the chloroform removed in vacuo at 40° C and the resulting paste left to rotate at 40° C overnight. The paste was redissolved in chloroform (200 ml) and stirred with calcium hydroxide (20 g) for 2 h, filtered through Celite® and then concentrated in vacuo. The resulting oil was purified by flash chromatography on silica gel (1:4 ethyl acetate:petrol) to afford compound 2 (3.7 g, 66%). 1H and 13C NMR were identical to that described in the literature (Evans & Grant, 1997).
2-[(2R,4aS,8aR)-2-(hydroxymethyl)-5,5,8a-trimethyl-decahydronaphthalen-1-yl] ethan-1-ol (4): Aluminium bromide (3.7 g, 13.8 mmol) was added portionwise to a solution of epoxy acetate 2 (3.7 g, 12.6 mmol) in anhydrous diethyl ether under an inert atmosphere. The reaction was stirred for 15 min and then quenched with aqueous sodium hydroxide (15% b.w., 50 ml). The diethyl ether solution was diluted further with diethyl ether (150 ml) and the organic layer was washed with water (50 ml), brine (50 ml), dried (MgSO4), and concentrated in vacuo to afford an oily solid which was used in the next step without purification.
The oily residue was redissolved in THF (50 ml) and stirred overnight with lithium aluminium hydride (1 g, excess) under an inert atmosphere. The next morning the reaction was deemed to be complete by TLC and quenched with water (1 ml), aqueous sodium hydroxide (15% b.w., 1 ml), and water (3 ml), filtered through Celite® and concentrated in vacuo to afford a solid residue. Purification of the crude residue was achieved by chromatography on silica gel (2:3 ethyl acetate:petrol) to afford diol 4 (1.80 g, 56%) as a crystalline solid. 1H NMR was identical to that previously described in the literature (Vlad et al., 1978). 13C NMR (125 MHz, CDCl3) δ 65.0, 64.5, 54.9, 48.1, 42.3, 40.6, 39.1, 38.4, 33.4, 33.3, 30.5, 29.6, 21.9, 21.6, 18.8, 14.0.
(4aR,6aS,10aR,10bS)-7,7,10a-Trimethyl-octahydro-1H-naphtho[2,1-c]pyran (Pyamber): p-TsOH.H2O (1.6 g, 8.4 mmol) was added portionwise to a stirred suspension of 4 (1.8 g, 7.1 mmol) in anhydrous toluene under an inert atmosphere. The resulting mixture was stirred at reflux under Dean and Stark conditions for 2 h, cooled to ambient temperature and then stirred with solid NaHCO3. After 1 h the suspension was filtered and the filtrate concentrated in vacuo. The crude residue was purified by flash chromatography on silica gel (1:24 ethyl acetate:petrol) to afford pyamber (1.48 g, 88%) as a white crystalline solid, m.p. 52–53° C. 1H NMR was identical to that described in the literature (Vlad et al., 1983). 13C NMR (125 MHz, CDCl3) δ 74.2, 69.1, 55.5, 54.1, 42.4, 38.5, 36.3, 35.9, 33.5, 33.3, 29.4, 25.2, 21.9, 21.0, 18.8, 14.3. C16H28O requires C, 81.29; H, 11.94. Found C, 81.10; H, 11.69.
Refinement
After structural refinement it was noted that high angle data (d < 0.90 Å) had systematically Fo**2 << Fc**2; this reflected the poor profiles noticed for higher angle data, but may have also been affected by minor movement (some icing was noted). As a consequence data was limited to that within the d resolution of 0.90 Å. A further 8 reflections (one at low angle) within this shell were clearly outliers and so were excluded.
The methyl H atoms were constrained to an ideal geometry (C—H = 0.98 Å) with Uiso(H) = 1.5Ueq(C), but were allowed to rotate freely about the adjacent C—C bonds. All other H atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms with C—H distances of 0.99Å and Uiso(H) = 1.2Ueq(C).
Figures
Fig. 1.
Chemical synthesis of Pyamber
Fig. 2.
An ORTEP (Farrugia, 1999) view showing the asymmetric unit with 20% probabilility ellipsoids.
Fig. 3.
Mercury cell packing view (Macrae et al., 2008) showing the linking hydrogen bonds (dotted lines, Table 1). Symmetry (i) 1 - x, 1/2 + y, 1/2 - z.
Crystal data
| C16H28O | F(000) = 528 |
| Mr = 236.38 | Dx = 1.121 Mg m−3 |
| Orthorhombic, P212121 | Cu Kα radiation, λ = 1.54178 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 484 reflections |
| a = 7.3497 (2) Å | θ = 6.6–72.0° |
| b = 11.1642 (3) Å | µ = 0.50 mm−1 |
| c = 17.0758 (12) Å | T = 123 K |
| V = 1401.13 (11) Å3 | Needle, colourless |
| Z = 4 | 0.70 × 0.40 × 0.13 mm |
Data collection
| Rigaku Spider diffractometer | 1969 independent reflections |
| Radiation source: Rigaku MM007 rotating anode | 1823 reflections with I > 2σ(I) |
| Rigaku VariMax-HF Confocal Optical System | Rint = 0.033 |
| Detector resolution: 10 pixels mm-1 | θmax = 58.9°, θmin = 6.5° |
| ω–scans | h = −8→5 |
| Absorption correction: multi-scan (ABSCOR; Higashi, 1995) | k = −10→12 |
| Tmin = 0.687, Tmax = 1.0 | l = −12→18 |
| 5141 measured reflections |
Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.034 | w = 1/[σ2(Fo2) + (0.0509P)2 + 0.0905P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.091 | (Δ/σ)max < 0.001 |
| S = 1.06 | Δρmax = 0.14 e Å−3 |
| 1969 reflections | Δρmin = −0.12 e Å−3 |
| 158 parameters | Extinction correction: SHELXL, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 0 restraints | Extinction coefficient: 0.0066 (8) |
| Primary atom site location: structure-invariant direct methods | Absolute structure: Flack (1983), 780 Friedel pairs |
| Secondary atom site location: difference Fourier map | Flack parameter: −0.4 (4) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.40780 (17) | 0.74765 (10) | 0.29584 (7) | 0.0466 (4) | |
| C1 | 0.1359 (2) | 0.60896 (15) | 0.02512 (10) | 0.0351 (4) | |
| H1A | 0.0226 | 0.6177 | 0.0560 | 0.042* | |
| H1B | 0.1734 | 0.6899 | 0.0078 | 0.042* | |
| C2 | 0.0957 (2) | 0.53268 (15) | −0.04708 (10) | 0.0393 (4) | |
| H2A | 0.0458 | 0.4544 | −0.0305 | 0.047* | |
| H2B | 0.0030 | 0.5732 | −0.0797 | 0.047* | |
| C3 | 0.2675 (2) | 0.51266 (16) | −0.09533 (10) | 0.0390 (4) | |
| H3A | 0.3091 | 0.5906 | −0.1164 | 0.047* | |
| H3B | 0.2377 | 0.4604 | −0.1404 | 0.047* | |
| C4 | 0.4228 (2) | 0.45576 (14) | −0.04894 (10) | 0.0348 (4) | |
| C5 | 0.4558 (2) | 0.53078 (14) | 0.02654 (9) | 0.0313 (4) | |
| H5 | 0.4936 | 0.6113 | 0.0067 | 0.038* | |
| C6 | 0.6176 (2) | 0.48888 (15) | 0.07615 (9) | 0.0347 (4) | |
| H6A | 0.5872 | 0.4114 | 0.1012 | 0.042* | |
| H6B | 0.7242 | 0.4760 | 0.0417 | 0.042* | |
| C7 | 0.6663 (2) | 0.57965 (16) | 0.13907 (10) | 0.0368 (4) | |
| H7A | 0.7145 | 0.6529 | 0.1138 | 0.044* | |
| H7B | 0.7635 | 0.5461 | 0.1727 | 0.044* | |
| C8 | 0.5036 (2) | 0.61297 (15) | 0.19000 (10) | 0.0352 (4) | |
| H8 | 0.4655 | 0.5405 | 0.2202 | 0.042* | |
| C9 | 0.3432 (2) | 0.65343 (15) | 0.13913 (9) | 0.0322 (4) | |
| H9 | 0.3881 | 0.7234 | 0.1081 | 0.039* | |
| C10 | 0.2851 (2) | 0.55687 (14) | 0.07831 (9) | 0.0305 (4) | |
| C11 | 0.1914 (2) | 0.70176 (16) | 0.19186 (10) | 0.0408 (5) | |
| H11A | 0.0955 | 0.7387 | 0.1591 | 0.049* | |
| H11B | 0.1360 | 0.6347 | 0.2214 | 0.049* | |
| C12 | 0.2640 (3) | 0.79350 (16) | 0.24854 (11) | 0.0462 (5) | |
| H12A | 0.3090 | 0.8637 | 0.2189 | 0.055* | |
| H12B | 0.1638 | 0.8209 | 0.2829 | 0.055* | |
| C13 | 0.5560 (2) | 0.71069 (16) | 0.24787 (10) | 0.0422 (5) | |
| H13A | 0.6555 | 0.6809 | 0.2818 | 0.051* | |
| H13B | 0.6026 | 0.7808 | 0.2186 | 0.051* | |
| C14 | 0.5941 (3) | 0.46036 (17) | −0.10071 (10) | 0.0472 (5) | |
| H14A | 0.6897 | 0.4108 | −0.0773 | 0.071* | |
| H14B | 0.6367 | 0.5433 | −0.1047 | 0.071* | |
| H14C | 0.5649 | 0.4300 | −0.1531 | 0.071* | |
| C15 | 0.3821 (3) | 0.32245 (14) | −0.03348 (11) | 0.0431 (5) | |
| H15A | 0.3920 | 0.2775 | −0.0826 | 0.065* | |
| H15B | 0.2586 | 0.3142 | −0.0125 | 0.065* | |
| H15C | 0.4698 | 0.2909 | 0.0045 | 0.065* | |
| C16 | 0.2115 (2) | 0.44509 (14) | 0.12111 (10) | 0.0372 (4) | |
| H16A | 0.1403 | 0.3964 | 0.0845 | 0.056* | |
| H16B | 0.1338 | 0.4702 | 0.1648 | 0.056* | |
| H16C | 0.3135 | 0.3978 | 0.1412 | 0.056* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0496 (8) | 0.0512 (7) | 0.0389 (6) | 0.0050 (7) | −0.0014 (6) | −0.0043 (6) |
| C1 | 0.0215 (10) | 0.0358 (9) | 0.0480 (10) | 0.0030 (8) | 0.0000 (8) | 0.0003 (8) |
| C2 | 0.0313 (10) | 0.0395 (9) | 0.0473 (10) | 0.0030 (9) | −0.0102 (8) | 0.0012 (8) |
| C3 | 0.0339 (10) | 0.0411 (9) | 0.0421 (9) | 0.0012 (8) | −0.0040 (8) | −0.0042 (8) |
| C4 | 0.0247 (9) | 0.0394 (9) | 0.0404 (9) | −0.0013 (8) | 0.0005 (8) | −0.0033 (8) |
| C5 | 0.0241 (9) | 0.0292 (9) | 0.0406 (9) | −0.0025 (7) | 0.0020 (8) | 0.0030 (8) |
| C6 | 0.0232 (9) | 0.0392 (9) | 0.0417 (9) | 0.0015 (8) | 0.0016 (8) | 0.0005 (8) |
| C7 | 0.0257 (10) | 0.0428 (10) | 0.0418 (9) | 0.0005 (8) | −0.0036 (8) | 0.0030 (8) |
| C8 | 0.0286 (9) | 0.0396 (10) | 0.0373 (9) | −0.0022 (8) | −0.0012 (7) | 0.0039 (8) |
| C9 | 0.0254 (9) | 0.0318 (9) | 0.0392 (9) | −0.0021 (7) | 0.0031 (7) | 0.0009 (8) |
| C10 | 0.0214 (9) | 0.0314 (9) | 0.0389 (9) | −0.0025 (7) | 0.0001 (8) | 0.0024 (7) |
| C11 | 0.0376 (11) | 0.0419 (10) | 0.0429 (9) | 0.0021 (9) | 0.0034 (9) | −0.0005 (9) |
| C12 | 0.0456 (11) | 0.0461 (11) | 0.0468 (10) | 0.0047 (10) | 0.0031 (10) | −0.0040 (9) |
| C13 | 0.0409 (11) | 0.0449 (11) | 0.0407 (9) | 0.0009 (9) | −0.0022 (10) | −0.0027 (9) |
| C14 | 0.0387 (11) | 0.0596 (12) | 0.0433 (10) | 0.0021 (10) | 0.0036 (9) | −0.0082 (9) |
| C15 | 0.0340 (11) | 0.0349 (9) | 0.0604 (11) | 0.0052 (8) | −0.0052 (10) | −0.0077 (9) |
| C16 | 0.0292 (10) | 0.0339 (9) | 0.0484 (10) | −0.0016 (8) | 0.0026 (8) | 0.0015 (8) |
Geometric parameters (Å, °)
| O1—C13 | 1.424 (2) | C8—C13 | 1.522 (2) |
| O1—C12 | 1.425 (2) | C8—C9 | 1.532 (2) |
| C1—C2 | 1.527 (2) | C8—H8 | 1.0000 |
| C1—C10 | 1.538 (2) | C9—C11 | 1.532 (2) |
| C1—H1A | 0.9900 | C9—C10 | 1.557 (2) |
| C1—H1B | 0.9900 | C9—H9 | 1.0000 |
| C2—C3 | 1.524 (2) | C10—C16 | 1.544 (2) |
| C2—H2A | 0.9900 | C11—C12 | 1.507 (2) |
| C2—H2B | 0.9900 | C11—H11A | 0.9900 |
| C3—C4 | 1.528 (2) | C11—H11B | 0.9900 |
| C3—H3A | 0.9900 | C12—H12A | 0.9900 |
| C3—H3B | 0.9900 | C12—H12B | 0.9900 |
| C4—C14 | 1.539 (2) | C13—H13A | 0.9900 |
| C4—C15 | 1.541 (2) | C13—H13B | 0.9900 |
| C4—C5 | 1.556 (2) | C14—H14A | 0.9800 |
| C5—C6 | 1.533 (2) | C14—H14B | 0.9800 |
| C5—C10 | 1.562 (2) | C14—H14C | 0.9800 |
| C5—H5 | 1.0000 | C15—H15A | 0.9800 |
| C6—C7 | 1.520 (2) | C15—H15B | 0.9800 |
| C6—H6A | 0.9900 | C15—H15C | 0.9800 |
| C6—H6B | 0.9900 | C16—H16A | 0.9800 |
| C7—C8 | 1.525 (2) | C16—H16B | 0.9800 |
| C7—H7A | 0.9900 | C16—H16C | 0.9800 |
| C7—H7B | 0.9900 | ||
| C13—O1—C12 | 110.20 (12) | C11—C9—C8 | 109.31 (13) |
| C2—C1—C10 | 113.79 (13) | C11—C9—C10 | 115.89 (13) |
| C2—C1—H1A | 108.8 | C8—C9—C10 | 112.64 (13) |
| C10—C1—H1A | 108.8 | C11—C9—H9 | 106.1 |
| C2—C1—H1B | 108.8 | C8—C9—H9 | 106.1 |
| C10—C1—H1B | 108.8 | C10—C9—H9 | 106.1 |
| H1A—C1—H1B | 107.7 | C1—C10—C16 | 109.57 (13) |
| C3—C2—C1 | 110.99 (13) | C1—C10—C9 | 109.12 (12) |
| C3—C2—H2A | 109.4 | C16—C10—C9 | 109.88 (12) |
| C1—C2—H2A | 109.4 | C1—C10—C5 | 108.01 (11) |
| C3—C2—H2B | 109.4 | C16—C10—C5 | 113.50 (13) |
| C1—C2—H2B | 109.4 | C9—C10—C5 | 106.64 (12) |
| H2A—C2—H2B | 108.0 | C12—C11—C9 | 111.05 (14) |
| C2—C3—C4 | 113.55 (13) | C12—C11—H11A | 109.4 |
| C2—C3—H3A | 108.9 | C9—C11—H11A | 109.4 |
| C4—C3—H3A | 108.9 | C12—C11—H11B | 109.4 |
| C2—C3—H3B | 108.9 | C9—C11—H11B | 109.4 |
| C4—C3—H3B | 108.9 | H11A—C11—H11B | 108.0 |
| H3A—C3—H3B | 107.7 | O1—C12—C11 | 112.49 (15) |
| C3—C4—C14 | 107.42 (13) | O1—C12—H12A | 109.1 |
| C3—C4—C15 | 110.21 (14) | C11—C12—H12A | 109.1 |
| C14—C4—C15 | 106.83 (14) | O1—C12—H12B | 109.1 |
| C3—C4—C5 | 108.80 (13) | C11—C12—H12B | 109.1 |
| C14—C4—C5 | 109.28 (13) | H12A—C12—H12B | 107.8 |
| C15—C4—C5 | 114.09 (14) | O1—C13—C8 | 112.80 (13) |
| C6—C5—C4 | 114.48 (12) | O1—C13—H13A | 109.0 |
| C6—C5—C10 | 111.54 (11) | C8—C13—H13A | 109.0 |
| C4—C5—C10 | 116.34 (12) | O1—C13—H13B | 109.0 |
| C6—C5—H5 | 104.3 | C8—C13—H13B | 109.0 |
| C4—C5—H5 | 104.3 | H13A—C13—H13B | 107.8 |
| C10—C5—H5 | 104.3 | C4—C14—H14A | 109.5 |
| C7—C6—C5 | 111.71 (13) | C4—C14—H14B | 109.5 |
| C7—C6—H6A | 109.3 | H14A—C14—H14B | 109.5 |
| C5—C6—H6A | 109.3 | C4—C14—H14C | 109.5 |
| C7—C6—H6B | 109.3 | H14A—C14—H14C | 109.5 |
| C5—C6—H6B | 109.3 | H14B—C14—H14C | 109.5 |
| H6A—C6—H6B | 107.9 | C4—C15—H15A | 109.5 |
| C6—C7—C8 | 112.41 (13) | C4—C15—H15B | 109.5 |
| C6—C7—H7A | 109.1 | H15A—C15—H15B | 109.5 |
| C8—C7—H7A | 109.1 | C4—C15—H15C | 109.5 |
| C6—C7—H7B | 109.1 | H15A—C15—H15C | 109.5 |
| C8—C7—H7B | 109.1 | H15B—C15—H15C | 109.5 |
| H7A—C7—H7B | 107.9 | C10—C16—H16A | 109.5 |
| C13—C8—C7 | 110.28 (13) | C10—C16—H16B | 109.5 |
| C13—C8—C9 | 110.59 (13) | H16A—C16—H16B | 109.5 |
| C7—C8—C9 | 110.61 (13) | C10—C16—H16C | 109.5 |
| C13—C8—H8 | 108.4 | H16A—C16—H16C | 109.5 |
| C7—C8—H8 | 108.4 | H16B—C16—H16C | 109.5 |
| C9—C8—H8 | 108.4 | ||
| C10—C1—C2—C3 | −56.49 (18) | C2—C1—C10—C9 | 168.00 (13) |
| C1—C2—C3—C4 | 56.36 (18) | C2—C1—C10—C5 | 52.44 (17) |
| C2—C3—C4—C14 | −170.86 (13) | C11—C9—C10—C1 | 58.17 (17) |
| C2—C3—C4—C15 | 73.11 (18) | C8—C9—C10—C1 | −174.92 (12) |
| C2—C3—C4—C5 | −52.67 (18) | C11—C9—C10—C16 | −61.98 (18) |
| C3—C4—C5—C6 | −175.92 (12) | C8—C9—C10—C16 | 64.93 (16) |
| C14—C4—C5—C6 | −58.92 (17) | C11—C9—C10—C5 | 174.60 (13) |
| C15—C4—C5—C6 | 60.59 (18) | C8—C9—C10—C5 | −58.48 (15) |
| C3—C4—C5—C10 | 51.59 (17) | C6—C5—C10—C1 | 174.96 (12) |
| C14—C4—C5—C10 | 168.59 (13) | C4—C5—C10—C1 | −51.21 (17) |
| C15—C4—C5—C10 | −71.91 (18) | C6—C5—C10—C16 | −63.35 (17) |
| C4—C5—C6—C7 | 168.28 (13) | C4—C5—C10—C16 | 70.48 (16) |
| C10—C5—C6—C7 | −56.98 (17) | C6—C5—C10—C9 | 57.78 (16) |
| C5—C6—C7—C8 | 53.70 (17) | C4—C5—C10—C9 | −168.39 (13) |
| C6—C7—C8—C13 | −175.75 (14) | C8—C9—C11—C12 | 50.94 (18) |
| C6—C7—C8—C9 | −53.10 (19) | C10—C9—C11—C12 | 179.51 (14) |
| C13—C8—C9—C11 | −50.14 (17) | C13—O1—C12—C11 | 60.46 (18) |
| C7—C8—C9—C11 | −172.61 (13) | C9—C11—C12—O1 | −56.93 (18) |
| C13—C8—C9—C10 | 179.51 (13) | C12—O1—C13—C8 | −60.03 (18) |
| C7—C8—C9—C10 | 57.04 (17) | C7—C8—C13—O1 | 178.54 (14) |
| C2—C1—C10—C16 | −71.65 (16) | C9—C8—C13—O1 | 55.88 (17) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C6—H6A···O1i | 0.99 | 2.54 | 3.474 (2) | 158 |
Symmetry codes: (i) −x+1, y−1/2, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: ZJ2024).
References
- Allen, F. H. (2002). Acta Cryst. B58, 380–388. [DOI] [PubMed]
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
- Cambie, R. C., Clark, G. R., Rickard, C. E. F., Rutledge, P. S., Ryan, G. R. & Woodgate, P. D. (1988). Aust. J. Chem. 41, 1171–1189.
- Evans, G. B. & Grant, P. K. (1997). Tetrahedron Lett. 38, 4709–4712.
- Farrugia, L. J. (1999). J. Appl. Cryst. 32, 837–838.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Grant, P. K., Chee, K. L., Prasad, J. S. & Tho, M. Y. (1988). Aust. J. Chem. 41, 711–725.
- Higashi, T. (1995). ABSCOR Rigaku Corporation, Tokyo, Japan.
- Hooft, R. W. W., Straver, L. H. & Spek, A. L. (2008). J. Appl. Cryst. 41, 96–103. [DOI] [PMC free article] [PubMed]
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Rigaku (1998). PROCESS-AUTO Rigaku Corporation, Tokyo, Japan.
- Rigaku (2005). CrystalClear: Rigaku Americas Corporation, The Woodlands, Texas, USA.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Vlad, P. F., Koltsa, M. N., Ungur, N. D., Dragalina, G. A. & Panasyuk, T. E. (1983). Chem. Heterocycl. Compd, 19, 253–258.
- Vlad, P. F., Ungur, N. D. & Koltsa, M. N. (1978). J. Gen. Chem. USSR, 48, 1776–1779.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811038694/zj2024sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811038694/zj2024Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536811038694/zj2024Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report