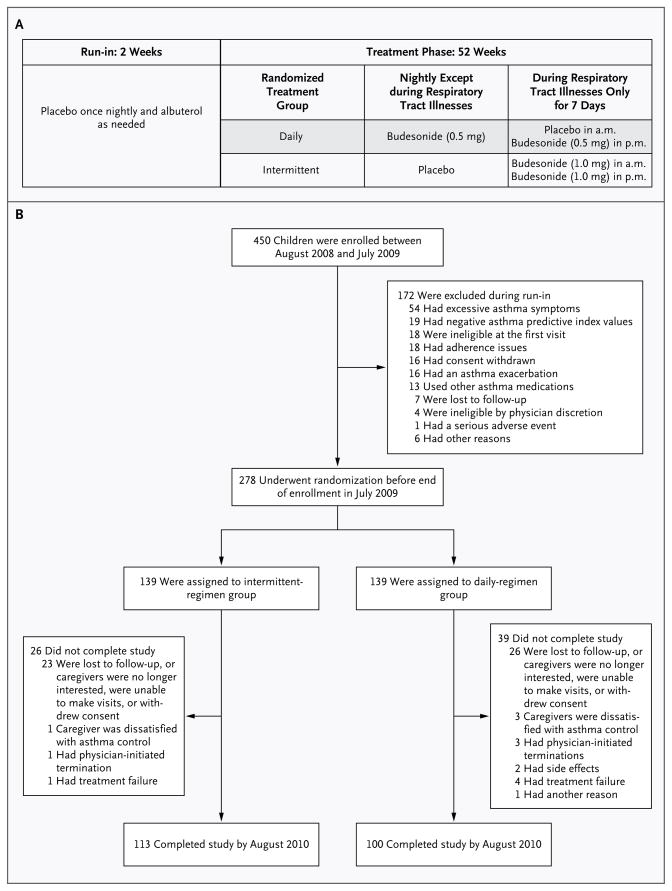
Figure 1. Study Design and Enrollment.
Panel A shows the study design and treatments. Intermittent high-dose nebulized budesonide inhalation suspension was administered at a dose of 1 mg twice daily in the form of Pulmicort Respules for 7 days at the onset of a predefined respiratory tract illness. A matched placebo was administered once nightly on all other days. Daily low-dose nebulized budesonide inhalation suspension was administered at a dose of 0.5 mg once nightly, also in the form of Pulmicort Respules. During respiratory tract illnesses, an appropriately matched morning placebo was used for 7 days. To maintain blinding during respiratory tract illnesses, daily treatments were discontinued for 7 days and respiratory illness kits that were based on the study-group assignment were administered for 7 days. After 7 days, regular daily treatments were restarted. Open-label rescue albuterol was administered per protocol during a respiratory tract illness and as needed. Study medications were administered with the use of a Pari Ultra II compressor with a Pari LC Sprint reusable nebulizer and a mask (Bubbles the Fish II or Pari Baby mask), if needed, or a mouthpiece, depending on the age of the child. Rescue albuterol was administered at a dose of 180 μg per treatment by metered-dose inhalation (Ventolin HFA, GlaxoSmithKline) through AeroChamber Z-STAT Plus with FlowSIGnal Whistle with ComfortSeal Mask (Monaghan Medical) or a solution of 2.5 mg of albuterol per treatment by nebulization according to protocol during a respiratory tract illness (four times daily, while the child was awake, for the first 48 hours) and as needed. Panel B shows the numbers of patients who were enrolled in the study, underwent randomization, and completed the study.