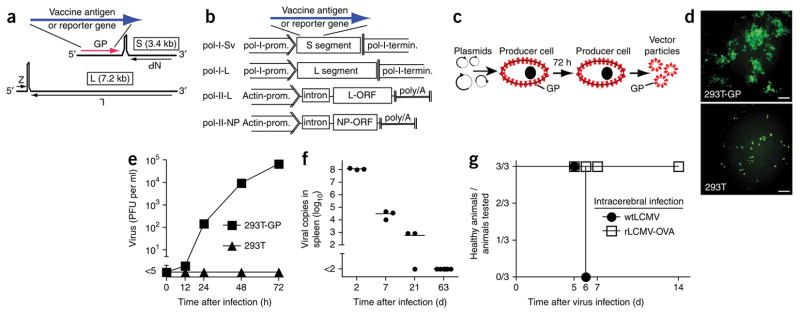
Figure 1.
Generation of rLCMV vectors, replication in cultured cells and rapid elimination and lack of pathogenicity in vivo. (a) Wild-type LCMV with its two genome segments (S and L) and the four open reading frames (ORFs). Substitution of GP for a vaccine antigen or reporter gene creates the rLCMV vectors. (b) Expression cassettes of plasmids used for the recovery of rLCMV vectors. In pol-I-Sv and pol-I-L10, the mouse polymerase I promoter (pol-I-prom.) and terminator (pol-I-termin.) drive intracellular expression of the LCMV vector S segment and L segment, respectively; pol-II-L10 and pol-II-NP10 express the respective viral proteins under control of an actin promoter (Actin-prom.)-driven expression cassette with intron and polyadenylation (poly(A)) signal. ORF, open reading frame. (c) Process to recover rLCMV vectors in producer cells. LCMV-GP protein (GP) on the surface of producer cells is incorporated into vector particles. (d) Fluorescence microscopy of GFP expression in 293T cells and GP-expressing 293T cells (293T-GP) infected for 48 h with rLCMV-GFP at a multiplicity of infection of 0.05. Scale bars, 100 μm. (e) Infectious progeny particles in supernatants of the 293T cells and GP-expressing 293T cells in d at 0–72 h after infection. Data are representative of three individual culture wells (mean ± s.e.m.). (f) Real-time RT-PCR analysis of vector S segment copies, measured in total RNA from spleen of mice vaccinated with rLCMV-GFP. Each symbol represents an individual mouse; small horizontal lines indicate the mean. (f) Disease development in mice infected intracerebrally with wild-type LCMV or rLCMV-OVA (three mice per group). Animals displaying clinical signs of lymphocytic choriomeningitis were killed in accordance with the Swiss law for animal protection.