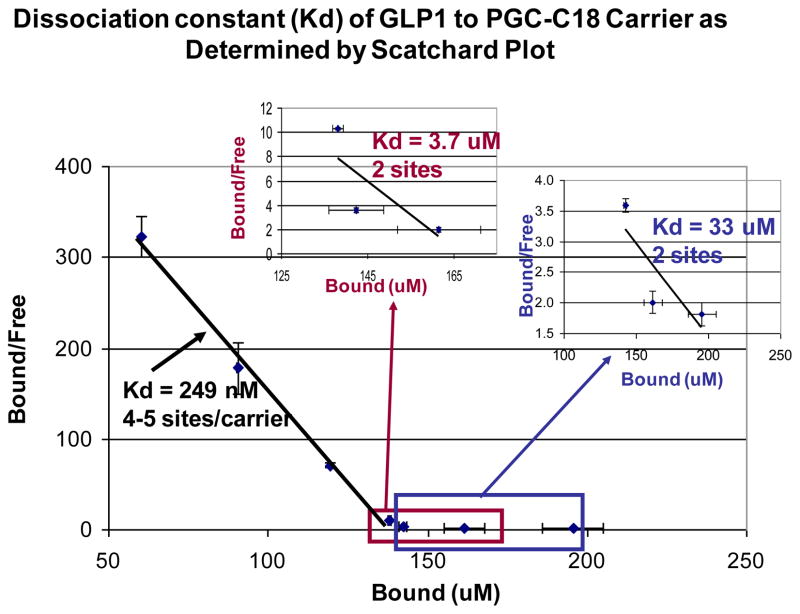
Fig. 3. Scatchard plot of GLP-1 binding to PGC-C18 shows various affinities.
In this particular experiment three Kd values (249 nM, 3.7 uM, and 33 uM) were calculated over three ranges of bound GLP-1 concentration. The % of GLP-1 bound to the carrier varies based on the available GLP-1. It is evident from this data that there is a range of interaction from high affinity (Kd of nM) to lower affinity interactions (Kd of uM) between the carrier and PGC-C18, and that a PGC-C18 capacity for GLP-1 of up to 9 is attainable. Those sites with average Kd of 249 nM have a capacity of 4–5 GLP-1(3kDa) molecules per molecule of PGC-C18 (350kDa). Those sites with Kd of 3.7uM have a capacity of 2 GLP-1 molecules per molecule of PGC-C18. Those sites with Kd of 33uM have a capacity of 2 GLP-1 molecules per molecule of PGC-C18. The Kd of the sites with the least affinity is very similar to the Km of DPP IV to GLP-1 (36uM), indicating that this site may not be a good sequestering site to protect GLP-1 from the DPP IV enzyme. In this particular experiment, 500uL samples were prepared in triplicate containing 0 or 5.0 mg carrier with 0.10, 0.15, 0.20, 0.25, 0.30, 0.40, and 0.50 mg of GLP-1 in PBS, pH 7.35. These correspond to 2%, 3%, 4%, 5%, 6%, 8% and 10% loading relative to carrier weight, respectively.