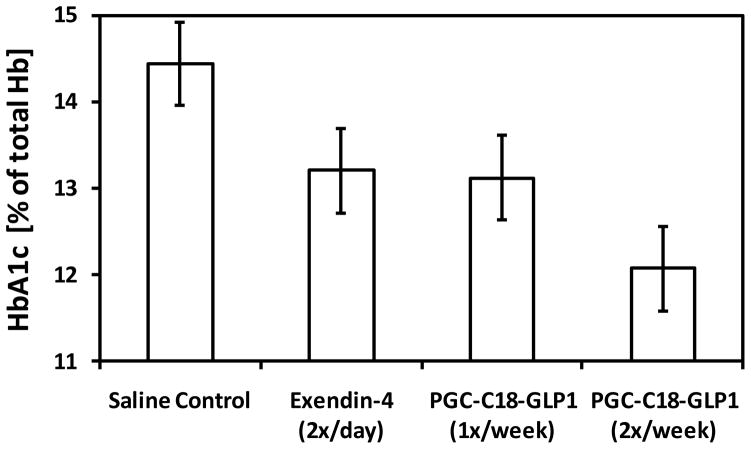
Fig. 7. GLP-1 formulated in PGC-C18 is effective in improving HbA1c level in ZDF rats.
Shown are HbA1c values (+ or − standard deviation) in ZDF rats (n=10) after 7 weeks of treatment. The Saline control group received once a day subcutaneous injections over 7 weeks. Exendin-4 2x/day group received twice a day subcutaneous injection, or 98 injections of exendin-4, at 3ug/kg over 7 weeks. The 1x/week GLP-1 group received once a week subcutaneous injections of 1mg/Kg of GLP-1 formulated in PGC-C18 or a total of 7 injections over 7 weeks. The 2x/week GLP-1 group received twice a week subcutaneous injections of 1mg/Kg of GLP-1 formulated in PGC-C18 or a total of 14 injections over 7 weeks. The GLP-1 formulated in PGC-C18 was at 2% loading or the ratio of GLP-1:PGC-C18 by weight is 1:50. The unformulated GLP-1 group received three times a week subcutaneous injections over 7 weeks (data not shown, not statistically significant from exendin-4 2x/day). HbA1c is a proxy for the average blood glucose level over the previous 3–5 weeks.