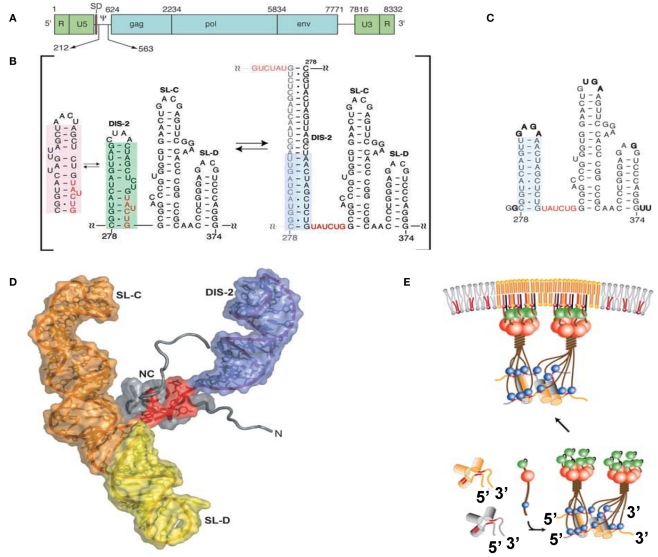
Figure 2.
(A) Genomic organization of MoMLV. (B) Secondary structure of the core encapsidation signal (ΨCES). Dimerization of DIS-2 induces a frame shift, exposing a UCUG element as a linker. (C) Secondary structure of mutant ΨCES that mimics the dimer-like conformation. (D) The complex of NC and mutant ΨCES. (E) Hypothetical mechanism for the genome packaging of MoMuLV. Upon dimerization, SL–CD exhibits a cross-kissing interaction, promoting the proximity of NC-binding sites. The SL–CD dimer functions as a scaffold that facilitates the Gag–Gag interaction. The ribonucleoprotein complex is transported to the plasma membrane. Reprinted with permission from [(A–D): D’Souza and Summers, 2004; (E): Miyazaki et al., 2010b].