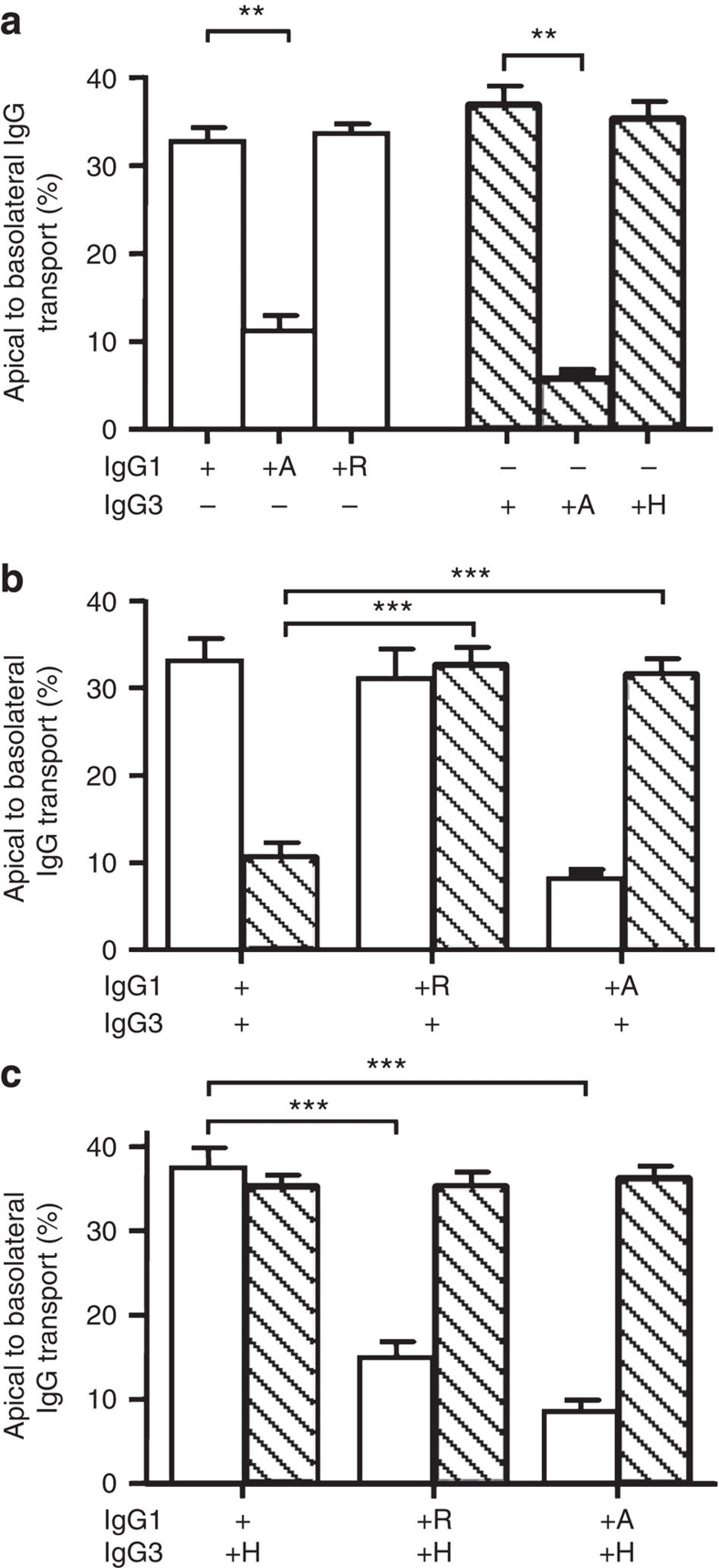
Figure 5. Inhibition of IgG3 transport by IgG1 is due to R435 in IgG3.
(a) Mutating the amino acid at position 435 in IgG1 (H435) and in IgG3 (R435) to an alanine reduces transport, while exchanging the histidine native to IgG1 and the arginine native to IgG3 on each others backbone had no effect on their transport rate when offered separately to FcRn-transfected A375 cells. (b) Whereas transport of IgG3-WT was inhibited in the presence of IgG1-WT, IgG1 bearing an alanine or an arginine at position 435 had no effect on IgG3 transport. (c) Transport of IgG3 with a histidine at position 435 was not inhibited by WT IgG1. When the amino acids found at position 435 in IgG1 and IgG3 were swapped, IgG1–H435R transport was inhibited by IgG3–R435H. (a–c) ± indicate the presence or absence of IgG (10 μg ml−1 per subclass), IgG1 is represented by open bars, IgG3 by hatched bars. The presence of mutated variants (435H, 435A and 435R) is indicated by the corresponding letter. The data represent mean and standard deviation from three independent experiments. Transport of WT IgG was compared with transport of mutant IgG by one-way ANOVA with Dunnett's multiple comparison test and significance. **P≤0.01; ***P≤0.001.