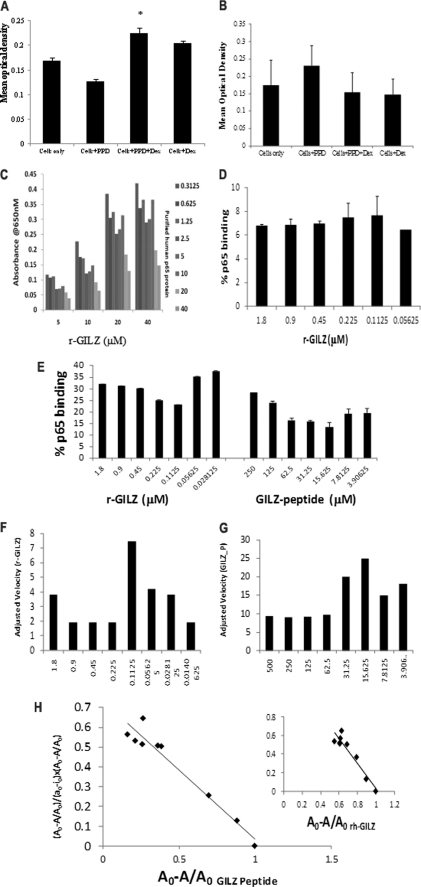
FIGURE 2.
GILZ-p65 binding analysis. CD4+ cells from the peripheral blood mononuclear cells of individuals vaccinated with the BCG vaccine were stimulated with PPD in the presence or absence of dexamethasone. Nuclear and cytoplasmic extracts were obtained from cells harvested after 24 h. Binding between the plate-bound r-p65 and the cytoplasmic GILZ (A) or the plate-bound r-GILZ and nuclear p65 (B) were detected by anti-GILZ (A) or anti-p65 (B) mAb, respectively. *, p < 0.05 when compared with cells stimulated with antigen alone. C, wells coated with r-GILZ at increasing concentrations (5–40 μm) were probed with full-length r-p65:DDK at increasing concentrations (0.3125–40 μm) and detected with anti-human GILZ mAb (D). High Bind ELISA plates coated with increasing concentrations of GILZ-P (3.9–250 mm) or r-GILZ (0.2–1.8 μm) were probed with 40 μm r-p65 (E) or r-p65ΔC14 (D) and detected with anti-DDK (E) and anti-p65 mAb (D) followed by trinitrobenzene substrate. Absorbance was read at 605 nm over a period of time between 0 and 300 s with a mixing time of 0.30 s and a 5-s interval between readings prior to stopping. The velocity of r-GILZ-p65 (F) and GILZ-P-p65 (G) reactions was measured as mean optical density/min. Scatchard plot analysis of bound p65 (A0 − A/A0) against the ratio of bound p65 to free r-GILZ/GILZ-P (y = (Ao − A/Ao)/(ao − io × Ao − A/Ao)) was used to determine the dissociation constant for the interaction between r-GILZ-p65 and GILZ-P and p65 (H).