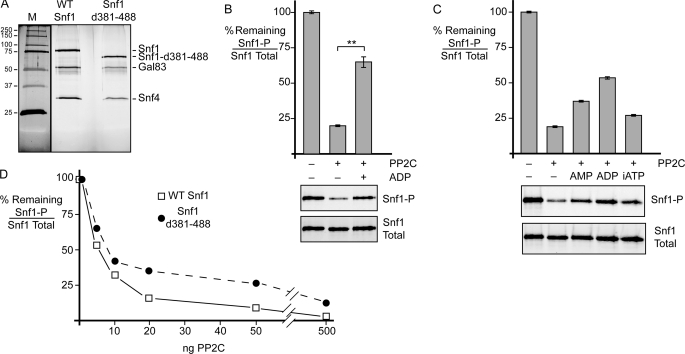
FIGURE 7.
Snf1 linker domain is not required for ligand-mediated protection in vitro or adenylate discrimination. A, SDS-polyacrylamide gel stained with silver nitrate showing the purity of the WT Snf1 heterotrimer and the heterotrimer with the linker deleted (Snf1Δ381–488 (Snf1-d381–488)). Protein size standards are shown on the left in kilodaltons (M). B, phosphatase protection assay of Snf1 heterotrimers with the linker (amino acids 381–488) deleted. Triplicate reactions were treated with PP2C with or without 0.8 mm ADP as shown. Mean values of the percentage of phosphorylated Snf1 (Snf1-P) to total Snf1 remaining are plotted, with error bars representing 1 S.E. Representative blots are shown below. C, ligand-mediated protection using the linker deletion mutant and different adenylate nucleotides present at 0.8 mm. Duplicate reactions were performed, and representative blots are shown below. Mean values are plotted, with error bars representing the range of duplicate values. iATP, non-hydrolyzable AMP-PNP. D, phosphatase resistance of wild-type Snf1 and Snf1Δ381–488. The percentage of phosphorylated Snf1 to total Snf1 remaining is plotted as a function of increasing concentrations of PP2C. **, p < 0.01.