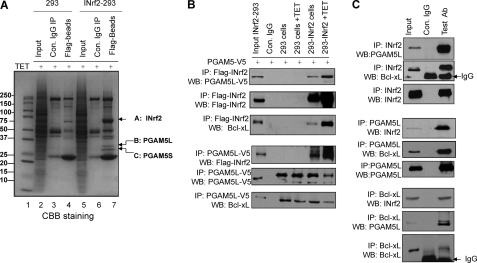
FIGURE 2.
INrf2 interacts with PGAM5-Bcl-xL complex. A, mass spectroscopic identification of PGAM5 proteins as interacting partners of INrf2. Control 293 cells and INrf2-293 cells were treated with tetracycline, and cell lysates were immunoprecipitated with anti-FLAG antibodies, immune complexes were separated by SDS-PAGE, and gels were stained with Coomassie Brilliant Blue. Gel slices containing bands indicated by arrows were reduced, alkylated, and digested with trypsin. Tryptic peptides were desalted and subjected to LC-MS/MS analysis. The Mascot software package was used to match the mass of the peptides with predicted tryptic peptides generated from the translated human genome. B, INrf2 interacts with PGAM5-Bcl-xL complex. Contro1 293 cells and INrf2-293 cells expressing tetracycline-inducible FLAG-INrf2 were transfected with PGAM5-V5 plasmid and treated with tetracycline. One mg of cell lysates was immunoprecipitated with anti-FLAG or anti-V5 antibodies and immunoblotted. C, endogenous INrf2 interacts with endogenous PGAM5L-Bcl-xL proteins. One mg of Hepa-1 cell lysates was immunoprecipitated with anti-INrf2 antibodies (top) or anti-PGAM5 antibodies (middle) or anti-Bcl-xL antibodies (bottom) and immunoblotted. All experiments were repeated three times.