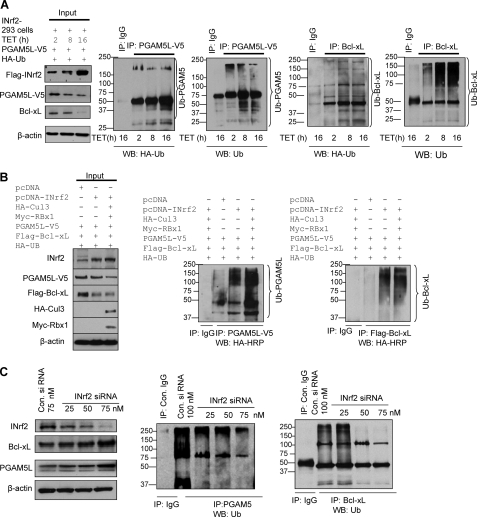
FIGURE 3.
INrf2-Cul3-Rbx1 complex ubiquitinates and degrades both PGAM5 and Bcl-xL proteins. A, INrf2-293 cells were co-transfected with PGAM5-V5 along with HA-ubiquitin plasmid and treated with tetracycline for different time periods. Sixty μg of proteins were immunoblotted with anti-FLAG, anti-V5, and anti-Bcl-xL antibodies (left). One mg of same cell lysates was immunoprecipitated with control IgG or anti-V5 or anti-Bcl-xL antibodies, and immune complexes were immunoblotted with anti-HA-HRP and anti-ubiquitin antibodies (right four panels). B, Hepa-1 cells were co-transfected with pcDNA-INrf2, PGAM5-V5, FLAG-Bcl-xL, HA-Cul3, Myc-Rbx1, and HA-ubiquitin in combinations as shown and immunoblotted (left). For visualization of ubiquitination of PGAM5 and Bcl-xL, the same one mg of lysates was immunoprecipitated with anti-V5 antibody and anti-FLAG antibody, respectively, and immunoblotted with anti-HA-HRP antibodies (right). C, effect of INrf2 siRNA on endogenous ubiquitination of PGAM5 and Bcl-xL. Hepa-1 cells were transfected with control (75 nm) or INrf2 siRNA and immunoblotted with indicated antibodies (left). The same one mg of lysates was immunoprecipitated with PGAM5 and Bcl-xL antibodies in separate experiments and immunoblotted with anti-ubiquitin (UB) antibodies (middle and right). All experiments were repeated three times.