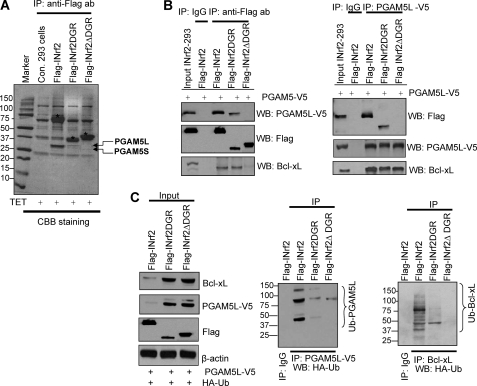
FIGURE 4.
INrf2-DGR domain is required for interaction and ubiquitination/degradation of PGAM5-Bcl-xL. A, tetracycline-induced expression of FLAG-INrf2, FLAG-INrf2DGR, and FLAG-INrf2ΔDGR in 293 cells. The cells were treated with tetracycline, and 10 mg of cell lysates were immunoprecipitated with anti-FLAG antibodies, and the immune complexes were separated by SDS-PAGE and stained with Coomassie Brilliant Blue. INrf2, INrf2DGR, and INrf2ΔDGR protein bands are labeled with asterisks, and interacting PGAM5 proteins are shown by arrows. B, INrf2DGR domain is required for interaction with PGAM5L-Bcl-xL. FLAG-INrf2-293, FLAG-INrf2DGR, and FLAG-INrf2ΔDGR cells were transfected with PGAM5-V5 plasmid and treated with tetracycline. One mg of lysates was immunoprecipitated with anti-V5 antibody (left) or anti-FLAG antibodies (right) and immunoblotted with anti-FLAG or anti-V5 or anti-Bcl-xL antibodies. C, INrf2DGR domain is essential for ubiquitination and degradation of PGAM5 and Bcl-xL protein. FLAG-INrf2, FLAG-INrf2DGR, and FLAG-INrf2ΔDGR-293 cells were co-transfected with PGAM5-V5 and HA-UB plasmids, treated with tetracycline, and immunoblotted with anti-FLAG, anti-V5, and anti-Bcl-xL antibodies (left). One mg of lysates was immunoprecipitated with anti-V5 or anti-Bcl-xL antibodies and immunoblotted with HA-HRP antibodies (right panels).