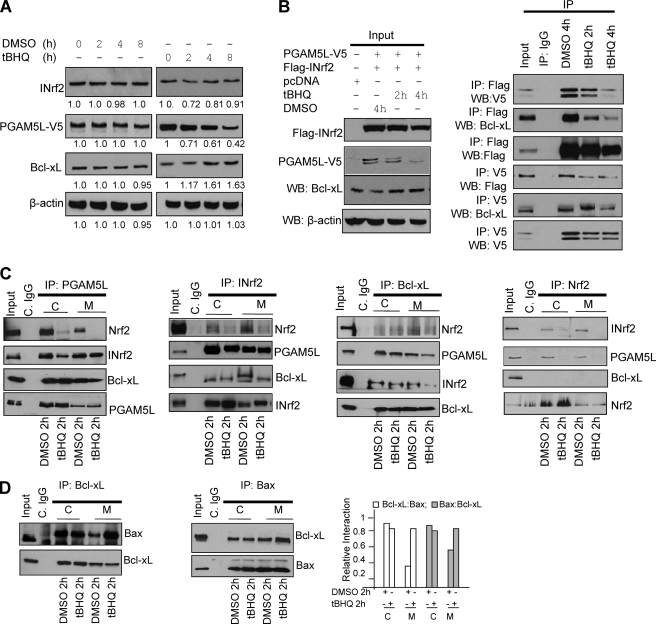
FIGURE 7.
Antioxidant t-BHQ destabilized Nrf2-INrf2-PGAM5-Bcl-xL complex in the cytosol and on the mitochondria, leading to the release of Bcl-xL. A, t-BHQ treatment leads to degradation of PGAM5 and stabilization of anti-apoptotic factor Bcl-xL. Hepa-1 cells transfected with PGAM5-V5 were treated with DMSO or t-BHQ (50 μm) for 2–8 h, lysed, and immunoblotted. Band intensities are shown below the immunoblots. B, t-BHQ treatment causes destabilization of Nrf2-INrf2-PGAM5-Bcl-xL complex and release of Bcl-xL. Hepa-1 cells were co-transfected with pcDNA or FLAG-INrf2 and PGAM5-V5 and treated with DMSO or t-BHQ (50 μm) for 2 and 4 h, lysed, and immunoblotted (left). One mg of the same cell lysates from transfected cells was immunoprecipitated with anti-FLAG or anti-V5 antibody, and the immune complexes were immunoblotted with anti-Bcl-xL, anti-FLAG or anti-V5 antibodies (right panels). C, t-BHQ causes Nrf2 release in the cytosol and Nrf2 and Bcl-xL release in the mitochondria. Hepa-1 cells were treated with DMSO or t-BHQ for 2 h, and 1 mg of cytosolic and 300 μg of mitochondrial lysates were immunoprecipitated with anti-PGAM5L, anti-INrf2, anti-Bcl-xL, and anti-Nrf2 antibodies and immunoblotted with the indicted antibodies (top panels). The band intensities of the respective panels are shown (bottom panels). C, cytosolic; M, mitochondrial. D, t-BHQ treatment increased heterodimerization of Bcl-xL and Bax protein on mitochondria. Hepa-1 cells were treated with DMSO or t-BHQ for 2 h, and 1 mg of cytosolic and 300 μg of mitochondrial lysates were immunoprecipitated with anti-Bcl-xL or anti-Bax antibodies and immunoblotted with indicted antibodies. All experiments were repeated two times, and one representative set of data is presented.