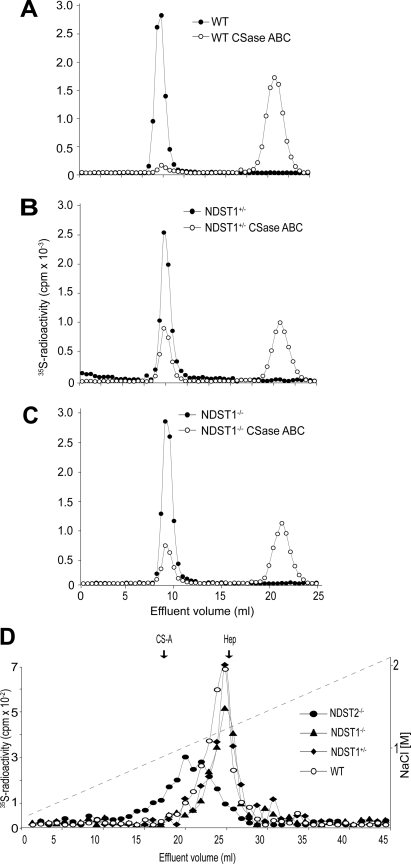
FIGURE 2.
Altered 35S-labeled heparin/HS production in NDST1-deficient MCs. A–C, analytical gel chromatography on Sephadex G50 of 35S-labeled glycosaminoglycans from WT (A), NDST1+/− (B), and NDST1−/− MCs (C) before (filled circles) and after treatment with chondroitinase ABC (CSase ABC; open circles). The column was eluted in 0.2 m NH4HCO3, and fractions of 0.5 ml were collected and analyzed for [35S] radioactivity. D, ion exchange chromatography on Mono Q of 35S-labeled heparin/HS isolated from WT (filled circles), NDST2−/− (filled diamonds), NDST1−/− (filled triangles), and NDST1+/− (open circles) as described under “Experimental Procedures.”. The samples were applied to a Mono Q column (Amersham Biosciences) equilibrated in 50 mm Tris-HCl, pH 8.0, 0.4 m NaCl and eluted in the same buffer with a gradient ranging from 0.4 to 2.1 m NaCl. The vertical arrows indicate the elution position of chondroitin sulfate (CS-A) and heparin (Hep) standards.