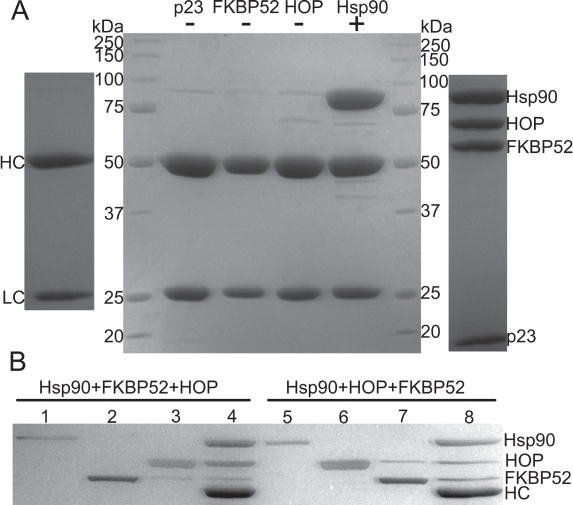
Figure 4. Co-immunoprecipitation experiments reveal the simultaneous binding interactions of Hsp90 with the two TPR domain-containing proteins FKBP52 and HOP.
(A) Hsp90-antibody specificity. Three negative controls (-) were performed in which the protein A Sepharose-Hsp90 antibody beads were incubated with 40μg the p23, FKBP52 and HOP proteins respectively. A positive control (+) incorporating 40μg Hsp90 into the antibody-bead mixture was also executed. Hsp90 was used as the bait protein for the subsequent experiments. Hsp90 antibody heavy (HC) and light (LC) chains (far left) as well as the anticipated distribution of the target proteins (far right). (B) The simultaneous binding of FKBP52 and HOP to Hsp90. Lanes 1-3 represent excess unbound protein in the initial washout fractions when Hsp90 (lane 1) was treated with a five-fold molar excess of FKBP52 (lane2) followed by a five-fold molar excess of HOP (lane 3) in a step-wise fashion. Lanes 5-7 represent a similar experiment in which HOP was added prior to FKBP52. Lanes 4 and 8 both represent noticeable amounts of Hsp902-FKBP521-HOP2 complex that were bound to the final antibody/Protein A-Sepharose bead pellets. Both the control experiments and the saturation experiments were resolved by 10% SDS-PAGE.