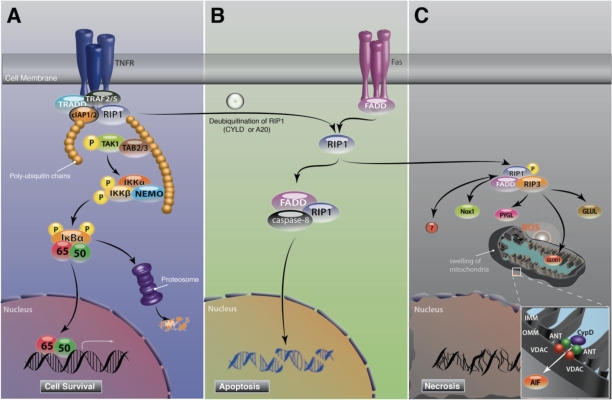
Figure 3. Schematic of RIP signaling pathway.
A. In response to TNF-α stimulation, RIP1 is recruited to TNFR and forms a membrane associated complex I with TRADD, TRAF2/5 and cIAP1/2, which in turn leads to polyubiquitination of RIP1 and pro-survival NF-κB activation.
B. RIP1 switches function to a regulator of cell death when RIP1 is unubiquitinated by A20 or CYLD. Deubiquitination of RIP1 leads to the formation of cytosolic DISC with FADD and caspase-8, the so-called complex II. In contrast to TNF signaling, Fas stimulation directly forms DISC. Activation of caspase-8 in DISC leads to apoptosis induction. During apoptosis, RIP1 is cleaved and inactivated by caspase-8.
C. In conditions where caspases are blocked or cannot be activated efficiently, RIP1 binds to RIP3, and both RIP1 and RIP3 kinases are phosphorylated at RIP1-RIP3 complex. RIP1 kinase phosphorylation is critical for necrosis induction. In response to TNF-α, RIP1 binds to NADPH oxidase 1 and produces superoxide. Activated RIP3 binds to PYGL, GLUL and GLUD1 and increases the production of mitochondrial ROS. ROS overproduction leads to mitochondrial dysfunction, resulting in the release of mitochondrial pro-death proteins.