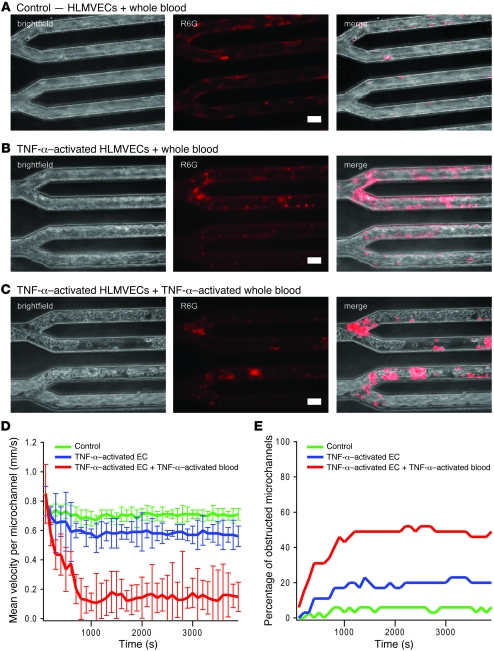
Figure 3. Decreased flow and microchannel occlusion due to activation with the inflammatory cytokine TNF-α.
(A) Brightfield imaging and epifluorescence using R6G, a fluorescent dye that preferentially stains leukocytes and platelets, revealed that when whole blood is flowed into the microdevice cultured with HLMVECs at postcapillary venular flow conditions, flow is steady overall, with occasional rolling but few adherent leukocytes. (B) When endothelial cells were activated with TNF-α before whole blood was flowed into the system, brightfield and R6G staining revealed a slight decrease in overall flow velocity, with occasional microchannel obstructions as well as an increase in adherent leukocytes. (C) TNF-α activation of both the endothelial cells and whole blood revealed a dramatic increase in microchannel obstruction, with subsequent decrease in overall flow likely due to a combination of increased adhesion and cell stiffness. Scale bars: 30 μm. (D) The addition of 0.5 μm fluorescent beads mixed into the whole blood samples enabled velocity quantification in each microchannel. The centerline bead velocity was measured for each channel and averaged for each data point. Compared with the control condition (no TNF-α activation), the average centerline velocity per microchannel (n = 32 microchannels/device) was significantly decreased when endothelial cells were preactivated with TNF-α (P < 0.05) and even more so when both endothelial cells and whole blood were preincubated with TNF-α (P < 0.005). Error bars show SD. (E) The percentage of microchannels completely obstructed due to aggregates of leukocytes increased with TNF-α of endothelial cells and increased even further with TNF-α activation of both the endothelial cells and whole blood.