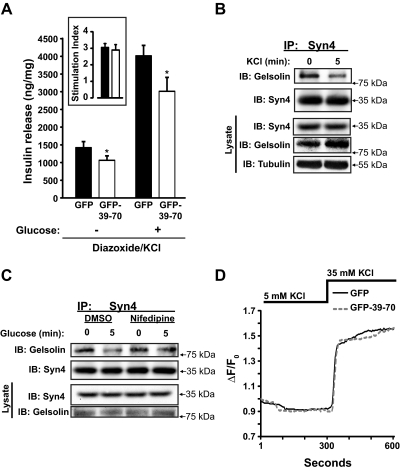
Fig. 8.
Effects of GFP-39–70 upon the triggering pathway. A, Effects of GFP-39–70 were evaluated by the diazoxide paradigm. MIN6 cells transduced to express GFP or GFP-39–70 proteins were incubated with 250 μm diazoxide and 35 mm KCl, followed by addition of 20 mm glucose for 30 min and upon insulin secretion quantified. Insulin released was normalized for corresponding cellular protein content (nanograms of insulin/milligrams of protein). Inset, Stimulation index = insulin release under diazoxide + KCl + glucose treatment/insulin release under diazoxide + KCl treatment alone (black bar, GFP; open bar, GFP-39–70). Data represent the mean ± se of at least three independent islet batches; *, P < 0.05 vs. GFP. B, MIN6 cells preincubated in MKRBB were stimulated 5 min with KCl (50 mm) and resultant cell lysates used for Syn4 immunoprecipitation (IP) as described in Fig. 1B. Data are representative of five independent experiments. C, MIN6 cells treated with vehicle [dimethylsulfoxide (DMSO)] or nifedipine for 1 h during preincubation in MKRBB were acutely stimulated with glucose (20 mm) for 5 min, in the continued presence of nifedipine/DMSO, and resultant cell lysates used in anti-Syn4 immunoprecipitation reactions. Protein abundances in starting input lysates (100 μg) were confirmed on a separate gel. Data are representative of three independent experiments. D, MIN6 cells on glass coverslips transduced to express GFP or GFP-39–70 proteins were preincubated in MKRBB supplemented with 2 mm glucose for 2 h followed by loading with fura 2-AM. Cells were subjected to perfusion for 300 sec under low KCl (5 mm) conditions and switched to buffer containing stimulatory KCl (35 mm) for an additional 300 sec to evaluate calcium influx. Each 340/380 ratio trace was normalized to the initial basal value (F0) of the respective trace to yield ΔF/F0. IB, Immunoblotting.