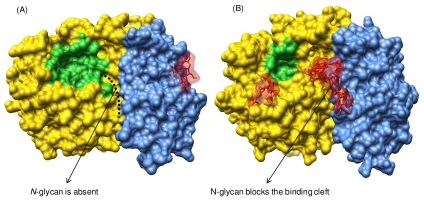
Figure 2. Three-dimensional structures of two glycosyl hydrolase 32 (GH32) family enzymes.
Surface representation of the overall 3D structure of (A) Arabidopsis thaliana cell-wall invertase (PDB database accession code: 2AC1) and (B) Cichorium intybus fructan 1-exohydrolase IIa (PDB database accession code: 1ST8). The N- and C-terminal domains are colored in yellow and blue, respectively. The attached N-glycan molecules are represented as sticks in red color. The active site is shown in green. Another binding pocket that extends between N- and C-terminal domains is orange, highlighted in (A). This cleft is reserved for higher DP-inulin type fructans. An open conformation of the mentioned cavity is observed in GH32 enzymes capable of degrading inulin substrates, such as C. intybus fructan 1-exohydrolase IIa (A). However, the introduction of a glycosyl chain blocks the cleft and prevents inulin binding and degradation in some GH32 enzymes, such as in A. thaliana invertase (B).