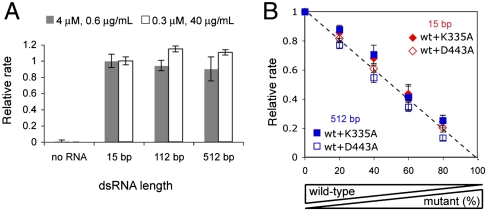
Fig. 3.
Intrinsic ATP hydrolysis activity of MDA5 is independent of filament length or catalytic activity of neighboring molecules. (A) Relative rates of ATP hydrolysis (mean ± SD, n = 3) of MDA5 (4 or 0.3 μM) with and without 15, 112, and 512 bp dsRNAs (0.6 or 40 μg/mL, equivalent to 0.16 or 10.7 μM MDA5 binding site), with respect to 15 bp rates. (B) Relative rates of ATP hydrolysis of mixtures of WT MDA5 and K335A or D443A at indicated ratios with total MDA5 concentration fixed at 0.3 μM. The protein mixture was incubated with 40 μg/mL (10.7 μM MDA5 binding site) of 15 or 512 bp dsRNA. Rates were normalized against that with 100% WT.