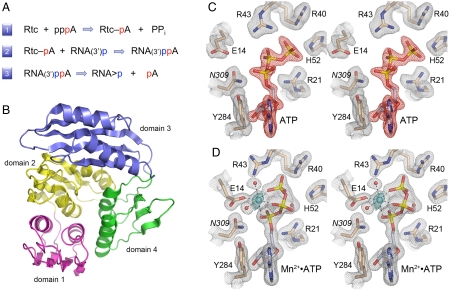
Fig. 1.
RNA 3′-phosphate cyclase: reaction pathway, tertiary structure, and ATP-binding modes. (A) The three chemical steps of the Rtc reaction pathway are shown: (1) Rtc adenylylation; (2) RNA adenylylation; and (3) cyclization. (B) The tertiary structure of E. coli RtcA (from pdb 3KGD) is shown as a ribbon trace with the four component domain modules colored as follows: N-terminal domain 1 in magenta (aa 4–85), domain 2 in yellow (aa 86–177), domain 3 in blue (aa 186–277), and domain 4 in green (aa 178–185 plus 278–339). Domains 1, 2, and 4 comprise a single globular unit with pseudo-threefold symmetry, into which domain 3 is inserted. (C and D) Stereo views of electron density maps of the active sites of the RtcA•ATP binary complex (C) and the RtcA•ATP•Mn2+ ternary complex (D). The gray meshes denotes 2Fo-Fc density of the refined models, contoured at 1.3σ. Amino acids and ATP are shown as stick models with beige and gray carbons, respectively. Waters are depicted as red spheres. The red mesh in (C) is an omit map (Fo-Fc) of the ATP density contoured at 3.5σ. The cyan mesh in (D) is the anomalous difference density for the manganese ion (cyan sphere) contoured at 8σ. Contacts to the ligands in the octahedral metal coordination complex are denoted by dashed lines in (D).