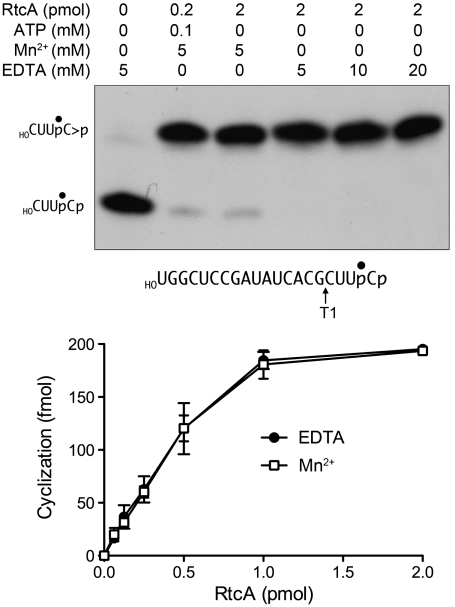
Fig. 5.
RNA cyclization by RtcA–AMP is metal-independent. (Upper) Cyclase reaction mixtures (10 μL) containing 50 mM Tris-HCl (pH 7.4), 2 mM DTT, 200 fmol of 32P-labeled 20-mer RNA substrate (prepared as described in SI Materials and Methods and depicted with the radiolabeled phosphate denoted by •), and RtcA, ATP, MnCl2 and/or EDTA as specified were incubated at 37 °C for 30 min. The samples were then digested for 15 min at 37 °C with 1,000 U RNase T1 (Fermentas). The samples were mixed with 10 μL of 90% formamide, 0.01% bromophenol blue/xylene cyanol, 5 mM EDTA and then resolved by electrophoresis (at 50 W constant power) through a 40-cm 20% polyacrylamide gel containing 8 M urea in 45 mM Tris-borate, 1.2 mM EDTA. The 32P-labeled RNAs were visualized by autoradiography of the gel. The positions of the substrate and product T1 fragments are indicated on the left. (Lower) Cyclase reaction mixtures (10 μL) containing 50 mM Tris-HCl (pH 7.4), 2 mM DTT, 200 fmol of 32P-labeled 20-mer RNA substrate, either 5 mM MnCl2 or 10 mM EDTA, and RtcA as specified were incubated at 37 °C for 30 min. Following RNase T1 digestion, the products were resolved by denaturing PAGE. The extents of conversion of the 3′-phosphate substrate into 2′,3′-cyclic phosphate product were quantified by scanning the gel with a Fuji Film BAS-2500 imager and are plotted as a function of input RtcA. Each datum is the average of three independent RtcA titration experiments ± SEM.