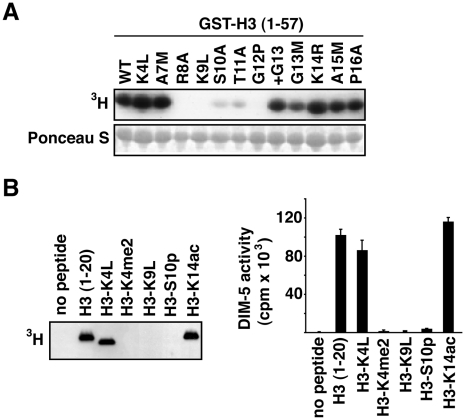
Figure 2. DIM-5 activity on histone H3 peptides.
(A) Residues surrounding H3K9 are critical for DIM-5 activity. Histone methyltransferase assays were performed with GST-H3 (1–57) bound to glutathione-agarose and S-adenosyl [methyl-3H]-L-methionine as the methyl group donor and analyzed by gel electrophoresis and fluorography. The membrane was stained with Ponceau S to confirm amount of protein loaded (bottom panel). (B) DIM-5 is sensitive to modifications of the H3 tail. In vitro assays were performed with unmodified H3 peptide (residues 1–20) or with peptides bearing covalent modifications (K4me2, S10ph and K14ac) or a substitution (K4L). Incorporation of the methyl group was analyzed by fluorography (left panel) or by liquid scintillation counting (right panel, each bar represents an average of three reactions).