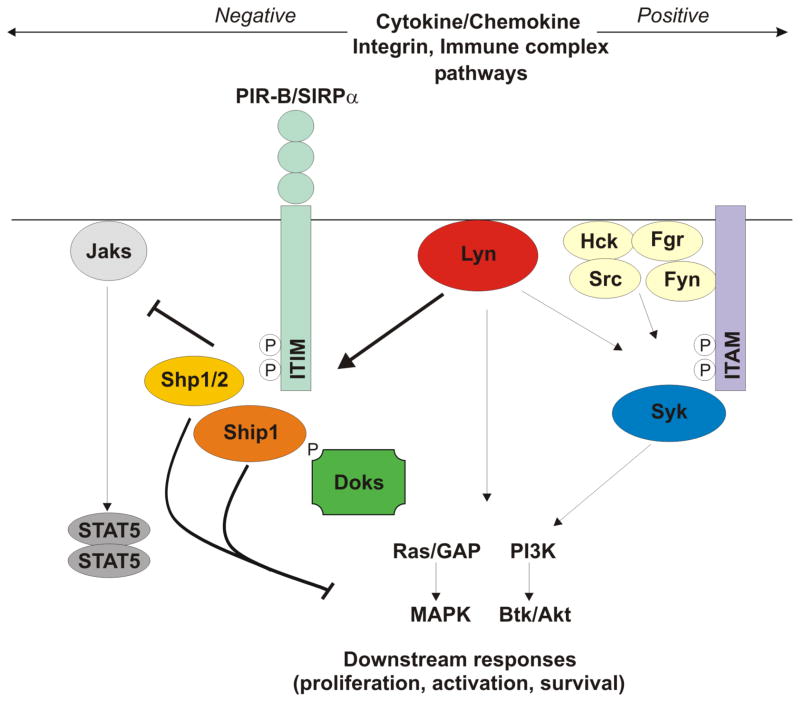
Figure 1. Proposed model of the dual role of Lyn in the modulation of signaling pathways in myeloid cells.
Lyn exerts its negative role in the modulation of signaling pathways in myeloid cells mainly by phosphorylating ITIM-containing inhibitory receptors, such as PIR-B and SIRPα thus recruiting inhibitory phosphatases, such as SHP1/2 or SHIP-1, which in turn downmodulate both proximal responses from Jak kinases and downstream responses at multiple sites distal to Ras. Additionally, Lyn can negatively modulate signaling responses in myeloid cells through the phosphorylation of the DOK family of cytoplasmic proteins that in turn recruit the Ras GTPase-activating protein (rasGAP) and SHIP-1 to down-modulate further signaling reactions. This inhibitory function of Lyn occurs especially in integrin, growth factor receptor and FcεRI signaling pathways. The mechanisms through which Lyn positively modulates signaling responses in myeloid cells are, on the other hand, less clear. Lyn can directly phosphorylate ITAM domains in various immunoreceptors associated signaling adapters (such as the FcRγ chain) leading to recruitment of Syk and activation of downstreamreactions. This positive regulatory function of Lyn occurs mainly in Fc signaling pathways. Lyn can also positively modulate signaling responses downstream to cytokine receptors, such as GM-CSF, IL-5, or chemokine receptors, but the mechanisms responsible for this phenomenon are not fully understood.