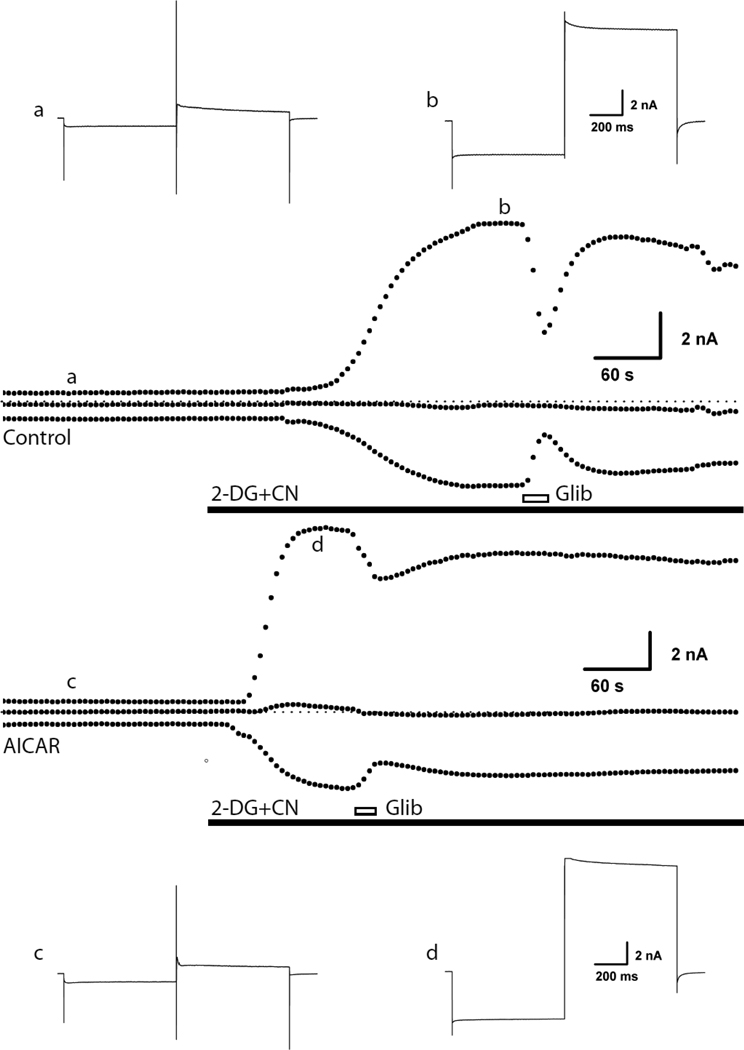
Fig 1.
Pretreatment of rat ventricular myocytes with AICAR shortened the time required for KATP channels to be activated by metabolic inhibition. Ventricular myocytes were voltage-clamped in whole-cell mode. From a holding potential of −70 mV, a dual voltage step was applied repeatedly (every 5s), consisting of a 700 ms hyperpolarization step to −100 followed by a 700 ms depolarization step to 0 mV. Two examples of currents recorded are shown at a collapsed time scale and each point represents a voltage clamp episode. For each recording, the top points are current at 0 mV, the middle are the holding current and the bottom points represent the current at −100 mV. The dotted lines represent the zero current level. The insets depict currents of individual voltage clamp episodes recorded at the points indicated. KATP channel current was activated with metabolic inhibition (2-deoxyglycose plus cyanide; 2-DG+CN) and blocked by glibenclamide (10µM; Glib). One of the cells was pre-incubated with AICAR (100 µM) for 10 min, whereas the control cell was perfused with Tyrode’s solution for an identical time. The adenosine receptor antagonist [8 (p-sulfophenyl) theophyline); 100µM] was included in the bath solution since AICAR may be a weak adenosine receptor agonist. Consistent with a previous report of incomplete sulfonylurea block of KATP channels during metabolic impairment [56], the current was only partially blocked by glibenclamide.