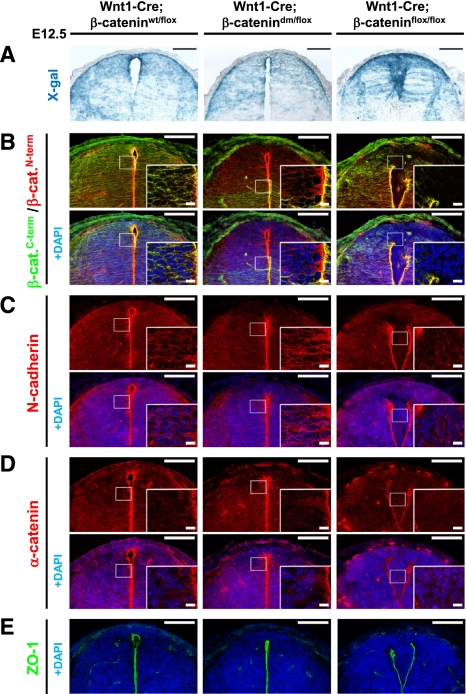
Figure 6.
β-Catenin-dm can fully restore the corrupted dorsal spinal cord morphology caused by defective adherens junctions in tissue lacking β-catenin. (A) X-gal staining visualizing Wnt1-Cre-mediated expression of β-galactosidase from R26R in the dorsal spinal cord of E12.5 embryos. Wild-type embryos (Wnt1-Cre/R26R; β-cateninwt/flox) and embryos expressing double-mutant β-catenin (Wnt1-Cre/R26R; β-catenindm/flox) display normal morphology and shape of the dorsal spinal cord. On the other hand, absence of β-catenin (Wnt1-Cre/R26R; β-cateninflox/flox) results in breakage of medial apical contacts and severe morphological defects. (B) Double-mutant β-catenin effectively restores the adhesion defects caused by the loss of β-catenin in Wnt1-Cre-positive tissues. Immunostaining with antibody recognizing either the N terminus or C terminus of β-catenin. The C terminus is missing in the double-mutant form, but protein expression is recognized by the antibody against the N terminus. No β-catenin is detected in the recombination-prone region of Wnt1-Cre; β-cateninflox/flox neural tubes. (C–E) N-cadherin (C), α-catenin (D), and ZO-1 (E) immunostainings for adherens and tight junctions do not show a significant difference between wild-type (Wnt1-Cre; β-cateninwt/flox) and mutant (Wnt1-Cre; β-catenindm/flox) spinal cord. Lack of β-catenin (Wnt1-Cre; β-cateninflox/flox) leads to adhesion defects associated with the breakdown of apical junctional complexes. Bar, 100 μm; bar in inset, 10 μm.