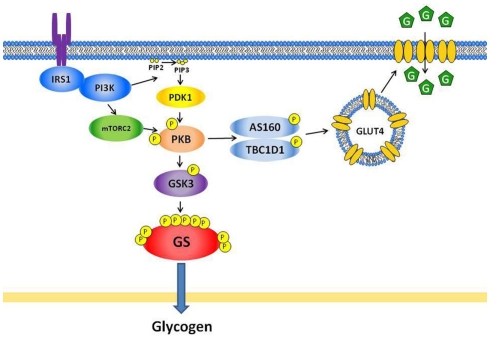
Figure 1.
Insulin signaling pathways regulating glucose transport and glycogen synthase in skeletal muscle. Insulin activates protein kinase B (PKB) through phosphatidylinositol 3-kinase (PI3K) and two upstream kinases; namely phosphoinositide-dependent protein kinase-1 (PDK1; phosphorylates PKB at threonine 308) and the mammalian target of rapamycin complexed with Rictor (mTORC2; phosphorylates PKB at serine 473). The activated PKB phosphorylates Akt substrate of 160 kDa (AS160, also called TBC1D4) and TBC1D1, which inhibits Rab GTPase activity and promotes GTP binding to Rabs, thereby allowing GLUT4 translocation. For glycogen synthesis, the activated PKB phosphorylates glycogen synthase kinase-3 (GSK3), which leads to inhibition of GSK3 activity and subsequently dephosphorylation and activation of glycogen synthase (GS). IRS, insulin receptor substrate; PIP2, phosphatidylinositol 4,5-biphosphate; PIP3, phosphatidylinositol 3,4,5-trisphosphate; G, glucose.