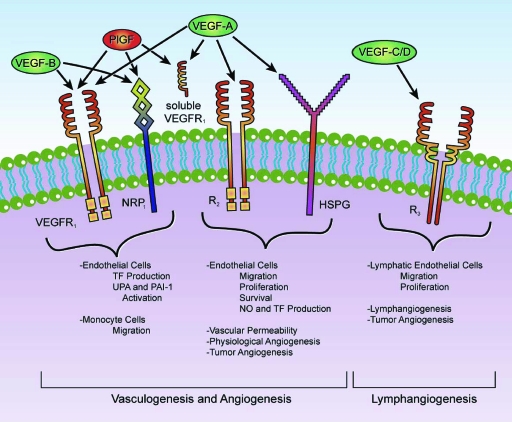
Figure 1.
VEGF-related family. VEGF165 is the predominant isoform and is commonly overexpressed in a variety of human solid tumors. VEGF-A expression is efficiently induced by hypoxia and regulates not only physiological but also most pathological angiogenesis, such as tumor angiogenesis. Free VEGF members exert their effects by binding a variety of cell-surface receptors including VEGFR-1, a 180-kDa transmembrane protein also called macrophage-colony stimulating factor receptor (fms)-like tyrosine kinase-1, or Flt-1, and VEGFR-2, a 200-kDa transmembrane protein also called kinase insert domain-containing receptor, or KDR [19]. A third structurally related tyrosine kinase receptor is the 180-kDa VEGFR-3, which is expressed broadly on endothelial cells during early embryogenesis but becomes restrictive to endothelial cells of adult lymphatic tissues and is necessary for adult lymphangiogenesis [57]. Two additional VEGFRs, NRP-1 and NRP-2, were also recently implicated in VEGF-mediated vascularization and lymphangiogenesis [58]. These receptors have a short intracellular domain and are not capable of signal transduction but may instead function as coreceptors for VEGFR-1 and VEGFR-2 to enhance their interaction with their respective ligands. An important preclinical trial revealed that blocking anti-NRP-1 antibodies has an additive effect with anti-VEGF therapy in reducing tumor growth [59]. Both VEGFR-1 and VEGFR-2 are members of the receptor tyrosine kinase superfamily, and they belong to the same subclass as receptors for platelet derived growth factors and fibroblast growth factors. VEGFRs have an extracellular domain composed of seven immunoglobulin-like regions that bind to VEGF, a single transmembrane region, and an intracellular tyrosine kinase domain [60]. Activation of these VEGFRs triggers the phosphorylation of a multitude of proteins that are active in signal transduction cascades. Some of the signaling pathways triggered by these mechanisms include the Akt/protein kinase B, endothelial nitric oxide synthase, mitogen-activated protein kinase, focal adhesion kinase, paxillin, Ras-Raf-mitogen-activated protein kinase/extracellular signal–related kinase kinase-extracellular signal–related kinase, and phospholipase C-γ pathways [61].
Abbreviations: HSPG, heparan sulfate proteoglycans; NO, nitric oxide; NRP, neuropilin; PAI-1, plasminogen activator inhibitor-1; PlGF, placental growth factor; TF, tissue factor; UPA, urokinase plasminogen activator; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.