TABLE 4.
Preparation and Cyclization of α-Aryl-α-diazo Ketones
| entry | α-diazo ketone | yield (%)a | product | yield (%) |
|---|---|---|---|---|
| 1 |

|
99 |

|
61b |
| 2 |

|
89 |
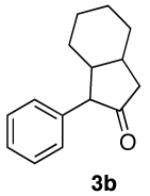
|
40d |
| 3 |

|
95 |

|
79 |
| 4 |

|
95 |

|
71b |
| 5 |

|
80 |

|
42b |
| 6 |

|
95 |

|
81b |
Yield of the diazo ketone.
Yield after equilibration of the epimeric products with DBU.
Previously reported ( Ref. 12).
The product is a mixture of ring fusion diastereomers.
