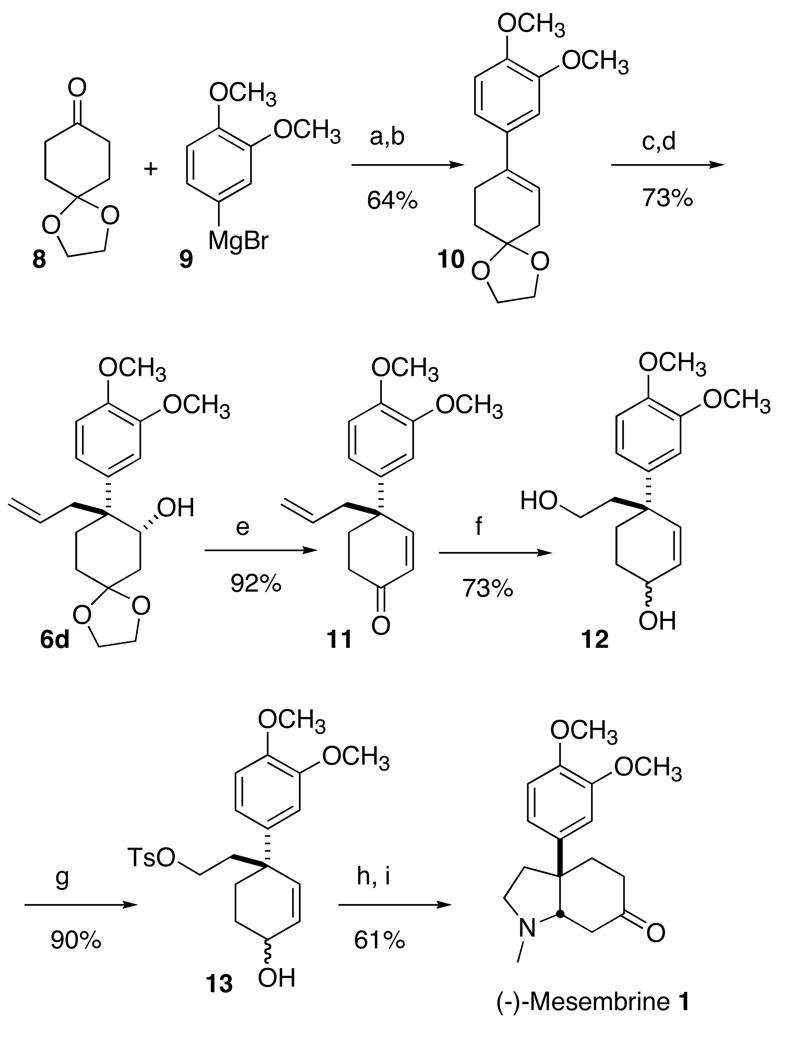
Scheme 2a.
a (a) THF, 0 °C to 20 °C, overnight; (b) benzene, PTSA (cat.), ethylene glycol (excess), reflux; (c) Shi's catalyst, DME-acetonitrile-H2O, 0 °C, 4 h; (d) allylmagnesium chloride, THF, 0 °C to 20 °C, overnight; (e) 10% aqueous HCl, THF, reflux, 1 h; (f) O3, MeOH, −78 °C; CeCl3·7H2O (1.0 equiv), NaBH4 (8.0 equiv), 0°C; (g) TsCl (1 equiv), Et3N, CH2Cl2, 20 °C, overnight; (h) 40% aqueous CH3NH2, THF, 65 °C, 1 h; (i) MnO2, CH2Cl2, 20 °C, 3 h.