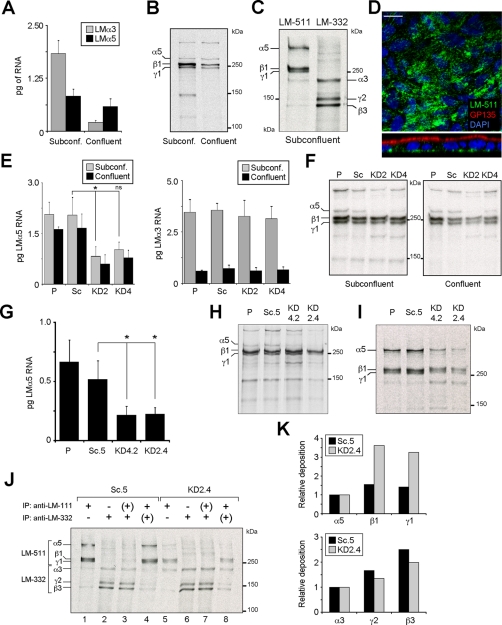
FIGURE 1:
Knockdown of LM-511 synthesis in MDCK cells. (A) Quantitative real-time PCR (qPCR) for LMα3 and LMα5 mRNA expression in either subconfluent (day 1) or confluent (day 4) MDCK cell cultures grown in growth medium on plastic. Bars represent the mean (±SD, n = 3). (B) Autoradiography of 35S-labeled and immunoprecipitated LM-511 (using an anti–LM-111 antibody) from subconfluent or confluent MDCK cell cultures. The experiment was repeated twice with similar results. (C) Representative autoradiography of two experiments of radiolabeled LM-511 and LM-332 extracted from ECM deposited by subconfluent MDCK cell cultures in serum-free, hormone-supplemented ExCell medium on collagen. (D) Confocal micrographs (XY, top; XZ, bottom) of confluent MDCK cell cultures stained for LM-511 using an anti–LM-111 antibody (green), GP135 (red), and nuclei (blue). No monospecific antibody against canine LMα5 is available. Scale bar, 10 μm. (E) qPCR for LMα5 (left) and LMα3 (right) expression (mean ± SD, n = 2) from parental MDCK cells (P), cells stably expressing scramble (Sc), or the two different LMα5 shRNA constructs (KD2 and KD4) cultured in growth medium on plastic. p = 0.0089 (multicomparison analysis); *p < 0.05; ns, not statistically significant. (F) Efficiency of LM-511 protein reduction, assessed by pulse labeling and immunoprecipitation using an anti–LM-111 antibody in both subconfluent and confluent KD2 and KD4 cell cultures compared with parental (P) and scramble (Sc). A representative autoradiography of two experiments is shown. (G) qPCR for LMα5 expression (mean ± SD, n = 2) of clones KD2.4, KD4.2, and Sc.5 and parental cells grown on collagen in ExCell. p = 0.0214 (multicomparison analysis); *p < 0.05. (H) Reduction of LM-511 protein biosynthesis and (I) deposition in the ECM of clones KD2.4 and KD4.2 compared with parental (P) and Sc.5 cell lines assessed by pulse-labeling and immunoprecipitation. (J) Reciprocal immunoprecipitation of LM-511 and LM-332 deposited into the ECM from radiolabeled subconfluent Sc.5 or KD2.4. Single immunoprecipitation of LM-511 (lanes 1 and 5) or LM-332 (lanes 2 and 6). From their respective unbound fractions (+) the reciprocal laminins were immunoprecipitated in a second round (lanes 3, 4, 7, and 8). (K) Deposition of β and γ laminin chains relative to α chains calculated from densitometry of lanes 1 and 2 (Sc.5) and lanes 5 and 6 (KD2.4) in J.