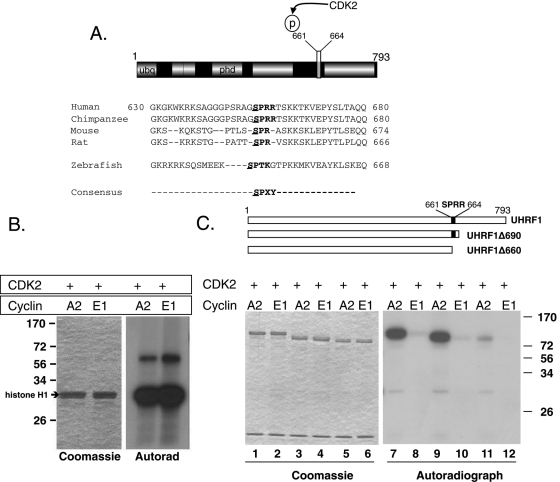
FIGURE 1:
UHRF1 is phosphorylated by CCNA2/CDK2 at Ser-661. (A) Domain structure of UHRF1 and alignment of the conserved CDK2 phosphorylation site in vertebrate UHRF1. Canonical consensus is bolded (SPXY: X = any amino acid; Y = basic amino acid). The putative phosphorylation residue is underlined. (B) Left, Coomassie-stained gel shows equal loading of histone H1. Right, autoradiograph of the phosphorylated histone H1. (C) Top, Schematic representation of UHRF1 truncation mutations used as substrates for CDK2 kinase assays. Black box indicates the CDK2 phosphorylation site (SPRR). Left, Coomassie staining showing equal loading in all lanes. CCNA2/CDK2 phosphorylates a truncated UHRF1 that retains the SPRR motif (lanes 7 and 9) but a truncated mutant lacking SPRR (lane 11) is barely phosphorylated. CCNE1/CDK2 has almost no kinase activity against UHRF1.