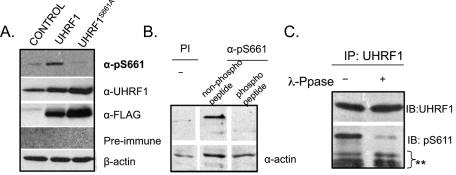
FIGURE 2:
α-pS661 detects phospho-UHRF1 in vivo and phosphorylation is eliminated when Ser-661 is changed to glycine. (A) HCT116 cells transfected with empty plasmid (CONTROL), FLAG epitope–tagged UHRF1 (UHRF1), or UHRF1 in which Ser-661 is changed to an alanine (UHRF1S661A). α-pS661 specifically recognizes a protein of appropriate size in FLAG-UHRF1 cells, but not in FLAG-UHRF1S661A cells, whereas both FLAG-UHRF1 and FLAG-UHRF1S661A are recognized by α-UHRF1 and α-FLAG. (B) Endogenous UHRF1 is phosphorylated on Ser-661. HCT116 cell lysates blotted with Preimmune (PI) or α-pS661 sera that was preincubated with either the phosphorylated or a cognate nonphosphorylated form of the immunizing peptide. Actin used as a loading control. (C) Lysates from HCT116 cells were immunoprecipitated with anti-UHRF1 antibody and then treated with or without λ-phosphatase. Immunoblotting (IB) with α-UHRF1 and with α-pS661 shows that phosphatase treatment reduces recognition of UHRF1 by α-pS661 but not by α-UHRF1.