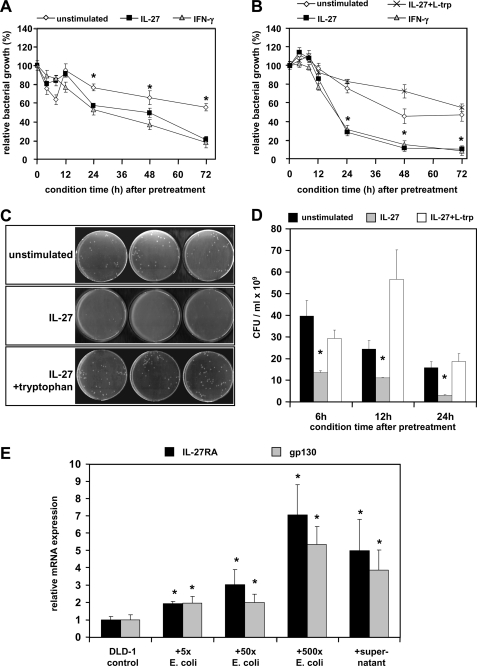
FIGURE 8.
IL-27 inhibits the growth of intestinal bacteria through tryptophan depletion via the induction of IDO1 enzymatic activity. A, E. coli K12 bacteria were incubated for 6–8 h in conditioned medium from IL-27 pretreated or untreated DLD-1 cells, and bacterial growth was determined photometrically at 600 nm. Medium from IL-27 pretreated cells conditioned for 24 h or longer exhibited a significant growth inhibiting effect on E. coli K12. IFN-γ (1000 units/ml) was used as a positive control. Bacterial growth rates were normalized to growth in fresh, untreated RPMI medium. *, p < 0.01 unstimulated versus IL-27 or versus IFN-γ. B, growth of E. coli WP2 (tryptophan auxotroph) was determined as in A. The IL-27-mediated significant bacteriostatic effect was more pronounced in strain WP2 in comparison with strain K12. The addition of l-tryptophan (10 μg/ml) completely reversed the bacterial growth-inhibiting effect of conditioned medium from IL-27-pretreated DLD-1 cells. *, p < 0.001 versus unstimulated or versus IL-27 + l-tryptophan. C and D, number of colony-forming units (cfu) per ml of bacterial cell suspension prepared as in A was significantly lower in conditioned medium from IL-27-pretreated cells in comparison with conditioned medium from untreated cells or when additional l-tryptophan was added. C is a representative picture of LB agar plates with a 10−9 dilution of the bacterial suspension grown in medium conditioned for 24 h. *, p < 0.01 versus unstimulated or versus IL-27 + l-tryptophan. E, expression of IL-27RA and gp130 in DLD-1 cells increased significantly when cells were co-cultured with E. coli bacteria for 6 h. The number of E. coli bacteria added was 5-, 50-, or 500-fold higher than the number of DLD-1 cells per well. A similar effect was achieved when sterile-filtered supernatant from an E. coli overnight culture was added to DLD-1 cells. Expression levels were normalized to the expression in untreated DLD-1 cells. *, p < 0.05 versus unstimulated.