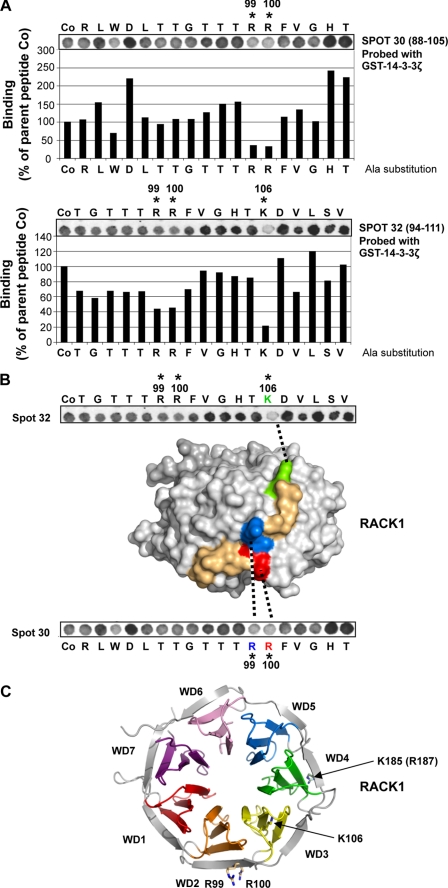
FIGURE 5.
Alanine-scanning array analysis of RACK1 peptides 30 and 32. A, arrays in which the 18 amino acids in RACK1-derived 18-mer peptides 30 and 32 (defined in Fig. 4B) were sequentially substituted with alanine were probed using GST-14-3-3ζ. The binding of GST-14-3-3ζ to each alanine-substituted RACK1 peptide was detected by anti-GST antibody and quantified by densitometry and is presented here as a percentage relative to the binding of GST-14-3-3ζ to the unsubstituted parent peptides (Co). n = 2. B, a surface rendition of the homologous A. thaliana RACK1A structure (9) with the cognate sequence (93AAGVSTRRFVGHTK106) shown colored reveals that the residues have prominent exposure on the edge of propeller blade WD2 and connecting loop to blade WD3. C, structure of RACK1A protein from A. thaliana (9) showing prominently surface-exposed side chains of basic residues Arg99, Arg100, and Lys106 (indicated by *) and cognate residue Lys185 (Arg187 in RACK1A) from the 14-3-3ζ binding locus on RACK1.