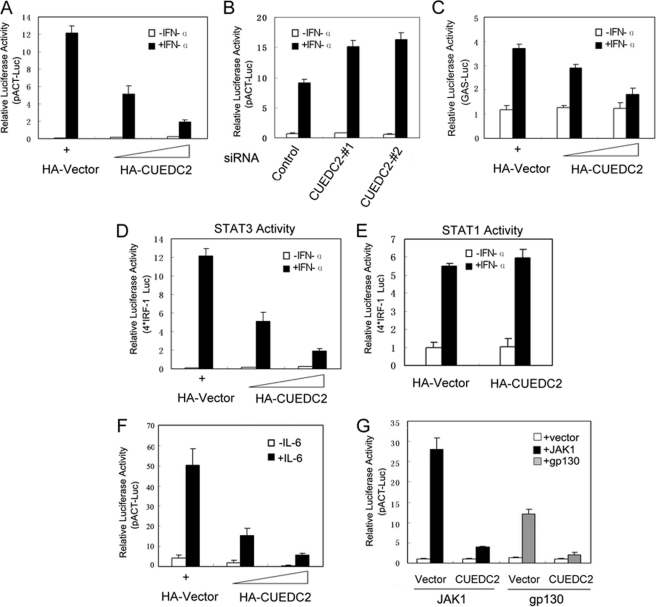
FIGURE 1.
CUEDC2 inhibits STAT3 transcriptional activity. A, HEK293T cells were transiently transfected in a 12-well plate with pACT luciferase reporter (200 ng), FLAG-STAT3 (200 ng), and increasing amounts of HA-CUEDC2 vectors (0, 200, and 500 ng) as indicated. 24 h after transfection, cells were stimulated with IFN-α (50 ng/ml) for an additional 6 h, and luciferase activity was measured. Renilla reporter pRL-TK (20 ng/well) vectors were used as an internal control for transfection efficiency. B, HEK 293T cells were transfected with control siRNA or the two different CUEDC2 siRNAs (20 nm) (#1 and #2). 24 h later pACT-Luc and FLAG-STAT3 plasmids were cotransfected as in A. After another 24 h, cells were treated with IFN-α for 6 h, and luciferase reporter assays were performed. C, HEK293T cells were transfected the same as in A, except the luciferase reporter gene was replaced by STAT3 responsive IFN-γ activating sequence reporter (500 ng/well). D and E, HEK293T cells were transfected with 4× IRF-1 luciferase reporter (500 ng/well) construct and STAT3 or STAT1(200 ng/well) together with or without CUEDC2 as indicated. Twenty-four hours after transfection, cells were left untreated (open columns) or treated with IFN-α (50 ng/ml) for 6 h, and luciferase activity was determined. F, HepG2 cells were transiently transfected with pACT-Luc (200 ng/well) together with increasing amounts of CUEDC2 vectors; 24 h after transfection cells were starved for 16–18 h in MEM with 0.5% serum then treated with IL-6 (100 ng/ml) for another 6 h, and luciferase activity was measured.G, JAK1 or glycoprotein 130 (gp130) expression vectors (200 ng/well) were co-transfected with pACT-Luc, FLAG-STAT3, and HA-CUEDC2 (or HA-vector) into HEK293T cells; 24 h later cells were harvested and detected for luciferase activity. All the results are the means ± S.E. of three independent experiments.