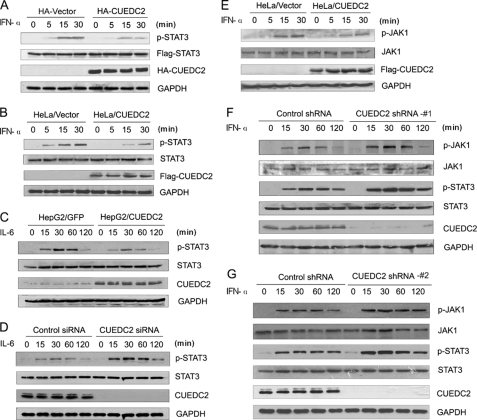
FIGURE 2.
CUEDC2 decreases STAT3 and JAK1 tyrosine phosphorylation. A, HEK293T cells in 6-well plates were transfected with FLAG-STAT3 (500 ng/well) together with HA-CUEDC2 or control vectors (1.5 μg/well). 24 h after transfection cells were stimulated by IFN-α (50 ng/ml) for the indicate periods, and total cell lysates were immunoblotted using anti-p-STAT3 (Tyr-705), anti-FLAG and anti-HA antibodies. B, HeLa/vector and HeLa/CUEDC2 cells were stimulated for 0–30 min with IFN-α (50 ng/ml), and endogenous STAT3 phosphorylation at Tyr-705 was detected by immunoblotting. C, HepG2 cells infected with retrovirus construction of pBabe-GFP(HepG2/GFP) or pBabe-CUEDC2(HepG2/CUEDC2) were starved for 16–18 h in MEM with 0.5% serum and then treated with IL-6 (100 ng/ml) for the indicated times. Cell lysates were analyzed by immunoblotting with indicated antibodies. D, HepG2 cells transfected with CUEDC2 siRNA(#1) or control siRNA were starved for 16–18 h in MEM with 0.5% serum and then treated with IL-6 (100 ng/ml) for the indicated times. Cell lysates were analyzed by immunoblotting, and STAT3 phosphorylation at Tyr-705 was detected. E, HeLa/vector and HeLa/CUEDC2 cells were treated the same as in B, phosphorylated JAK1 was detected by immunoblotting with anti-p-JAK1 (Tyr-1022/1023). F and G, cell extracts were prepared from HeLa cells with control or CUEDC2 shRNA (#1 or #2), then treated by IFN-α (50 ng/ml) for the indicated periods, and immunoblot analysis was performed.