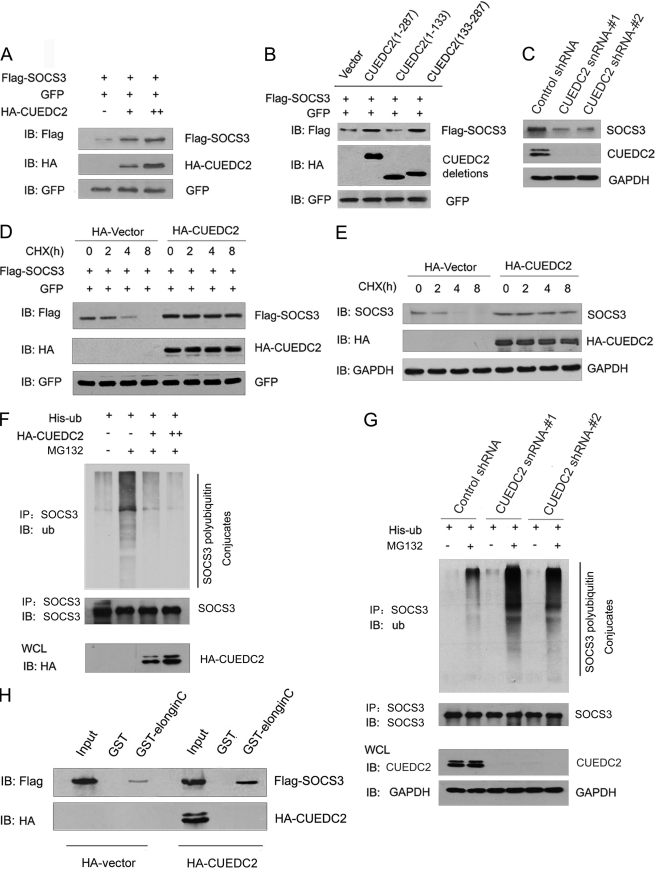
FIGURE 6.
CUEDC2 stabilizes SOCS3 by protecting it from degradation via enhancing SOCS3-Elongin C interaction. A, HEK293T cells were transfected with equal amounts of FLAG-SOCS3 (200 ng), GFP expression vector (50 ng), and increasing amounts of HA-CUEDC2 expression vectors (0, 0.5, and 1.5 μg). Cell lysates were subjected to immunoblotting (IB) using antibodies as indicated. Levels of GFP were shown as equal transfection efficiency. B, wild type or different deletions of CUEDC2 constructs were transfected into HEK293T cells, and the same assays were performed as in A. C, lysates from HeLa cells with control or CUEDC2 shRNA (#1 and #2) were subjected to immunoblotting using antibodies as indicated. D and E, HA-CUEDC2 or HA vectors were transfected into HeLa cells. 16 h after transfection, cells were treated with the protein synthesis inhibitor cycloheximide (CHX, 50 μg/ml), harvested at the indicated times, and then exogenous (D) and endogenous SOCS3 (E) protein levels were analyzed by immunoblotting. F, HeLa cells were transfected as indicated and treated with or without the proteasome inhibitor MG132 (10 μm) for an additional 6 h. Cell lysates were immunoprecipitated (IP) with anti-SOCS3 and detected with antibodies as indicated. ub, ubiquitin, G, HeLa cells with control or CUEDC2 shRNA (#1 and #2) were transfected and treated as indicated, and cell lysates were immunoprecipitated with anti-SOCS3 and detected with antibodies as indicated. H, HEK293T cells were cotransfected with HA-CUEDC2 (or HA-vectors) and FLAG-SOCS3, cell lysates were prepared, and input was normalized. After incubation with purified GST or GST-Elongin C fusion protein and extensive washes, bound proteins were analyzed by immunoblotting with anti-FLAG and anti-HA antibody. WCL, whole cell lysates.