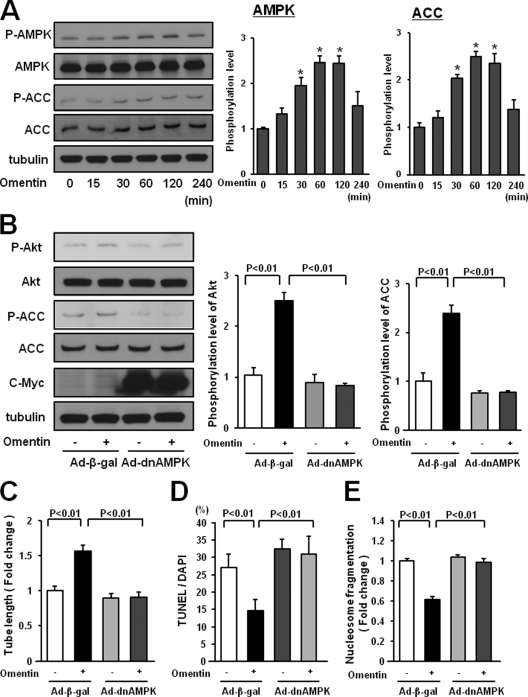
FIGURE 6.
AMPK activation is essential for endothelial cell responses to omentin. A, time-dependent changes in the phosphorylation of AMPK and ACC in endothelial cells following stimulation with omentin (left panels). Changes in the phosphorylation of AMPK (P-AMPK) and ACC (P-ACC) following omentin treatment were determined by Western blot analysis. Representative blots are shown. Relative phosphorylation levels of AMPK and ACC were quantified by using ImageJ software. Immunoblots were normalized to α-tubulin signal. Results are shown as the mean ± S.D. (n = 3). B, role of AMPK in regulation of omentin-induced Akt signaling. HUVECs were transduced with Ad-dnAMPK or Ad-β-gal 24 h before serum-starvation. After a 16-h serum starvation, cells were treated with omentin (300 ng/ml) for 60 min. P-Akt was determined by Western blot analysis. C, AMPK signaling is required for omentin-induced endothelial cell differentiation. HUVECs were transduced with Ad-dnAMPK or Ad-β-gal. After 18 h of serum starvation, a Matrigel assay was performed. Cells were treated with omentin (300 ng/ml) or vehicle. Quantitative analyses of the network tube length are shown. Results are shown as the mean ± S.D. (n = 5). D and E, Involvement of AMPK in omentin-induced endothelial cell survival. After transduction with Ad-dnAMPK or Ad-β-gal, cells were incubated in serum-free media for 48 h. Cells were treated with omentin (300 ng/ml) or vehicle. Apoptotic nuclei were identified by TUNEL staining, and total nuclei were identified by 4′,6-diamidino-2-phenylindole counterstaining. Quantitative analyses of the frequency of TUNEL-positive HUVECs are shown (D). Nucleosome fragmentation was determined by enzyme-linked immunosorbent assay (E). Results are shown as the mean ± S.D. (n = 5).