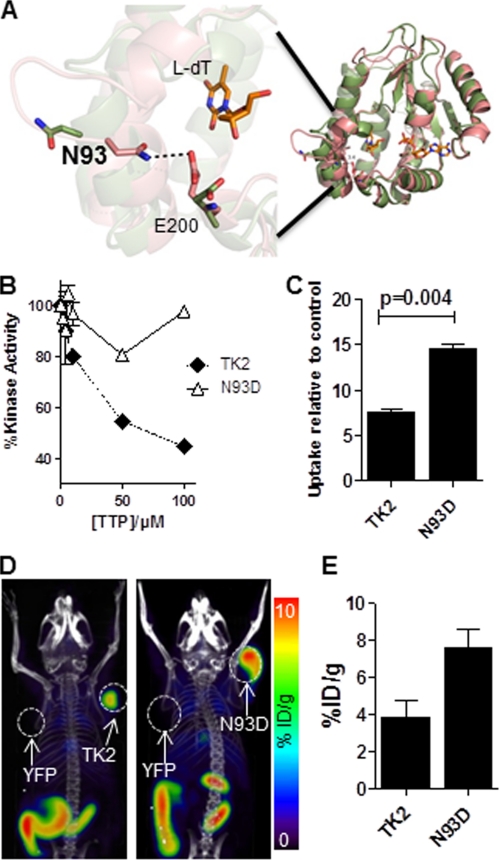
FIGURE 2.
Evaluation of the TK2-N93D mutant. A, model of WT TK2 bound with l-dT in both the closed (green) and open (pink) conformation of the enzyme. ADP is bound in the phosphate donor pocket shown in this model. The enzyme is active in the closed conformation, which is stabilized by bonds between residues Asn-93 and Glu-200. When asparagine 93 is mutated to a glutamine, the bonds are disrupted, and the enzyme is predicted to switch to an open (inactive) conformation. B, l-FMAU kinase assay using recombinant WT TK2 and TK2-N93D in the presence of increasing concentrations of dTTP is shown. C, l-18F-FMAU uptake assay using WT TK2- or TK2-N93D-expressing L1210 cells is shown. Probe uptake values are reported relative to a control L1210 cell line that expresses YFP. Results are for a representative experiment or n = 2 experiments. D, l-18F-FMAU microPET/CT scans of mice bearing L1210 tumors engineered to express various PRGs (TK2, L1210-TK2; N93D, L1210-TK2-N93D; YFP, L1210-YFP). E, shown is quantification of PET scans from panel D. %ID/g, % injected dose/g.