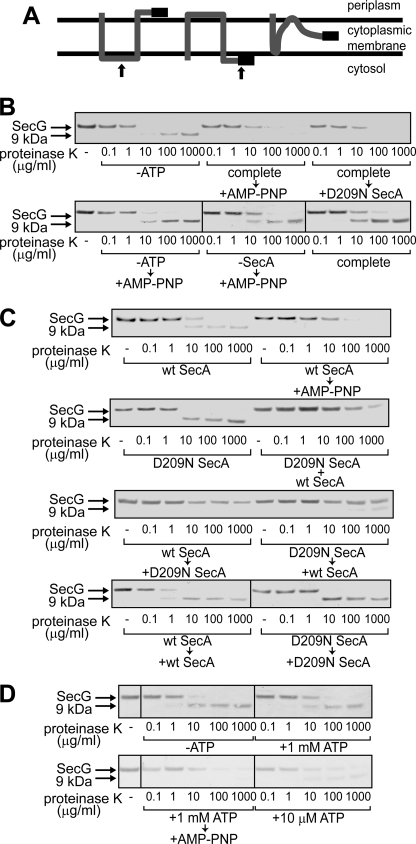
FIGURE 2.
Topology changes of SecG induced by D209N SecA. A, topology arrangement of SecG. SecG possesses two transmembrane stretches with its N and C termini being exposed to the periplasmic space (left). When inverted, both termini are exposed to the cytoplasmic side (center). At the right, the intermediate topology is shown. The black bar represents the antigenic region of the anti-SecG antiserum. The arrows at the left and center indicate the positions where proteinase K added outside of IMV cleaves SecG. B, addition of an excess amount of D209N SecA during proOmpA translocation caused SecG inversion. ProOmpA translocation into IMV prepared from K003 was carried out in the presence of 2 μg/ml WT SecA, as described under “Experimental Procedures” (complete). Where specified, ATP and SecA were omitted (-ATP and -SecA, respectively). At 5 min after initiation of the translocation reaction, either 20 mm AMP-PNP/MgSO4 (+AMP-PNP) or 20 μg/ml D209N SecA (+D209N SecA) was added as specified. After the reaction mixtures had been incubated for further 5 min, they were chilled on ice, followed by proteinase K digestion at the indicated concentrations. Samples were then analyzed by SDS-PAGE and immunoblotting using anti-SecG antiserum. The bands of SecG and the C-terminal 9 kDa are indicated by arrows. C, D209N SecA together with WT SecA causes the intermediate topology of SecG. The translocation reaction was initiated in the presence of WT SecA (wt SecA, wt SecA → AMP-PNP, wt SecA → D209N SecA, wt SecA → wt SecA), D209N SecA (D209N SecA, D209N SecA → wt SecA, D209N SecA → D209N SecA), or both (D209N SecA + wt SecA). The samples indicated by arrows received the indicated materials (20 mm AMP-PNP/MgSO4, WT SecA, or D209N SecA) 5 min after initiation of the reactions. The SecA concentration with one kind of SecA was 120 μg/ml, whereas that with two kinds was 60 μg/ml each. After all samples had been incubated at 37 °C for 10 min in total, they were chilled on ice, followed by proteinase K digestion and SDS-PAGE/immunoblotting as in B. D, a low concentration of ATP causes a change in the SecG topology. The proOmpA translocation reaction mixtures contained 60 μg/ml WT SecA and different concentrations of ATP (0 mm -ATP, 1 mm +1 mm ATP and complete → +AMP-PNP, and 10 μm +10 μm ATP). The reaction was allowed at 37 °C for 10 min. For the “complete → +AMP-PNP” sample, 20 mm AMP-PNP/MgSO4 was added at 5 min. Samples were processed as described in B and C.