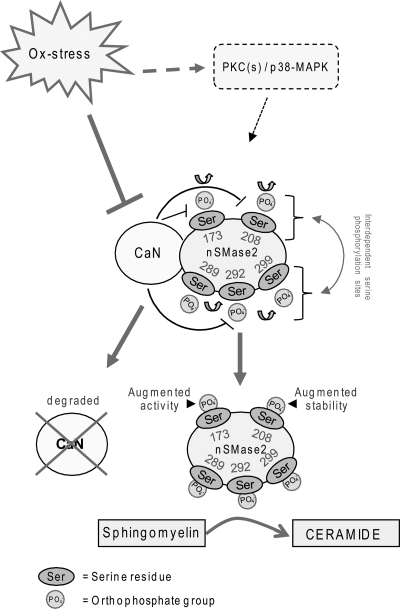
FIGURE 7.
Proposed model of nSMase2 phosphorylation machinery that affects its function. nSMase2 is constitutively phosphorylated on serine residues downstream of p38-MAPK (p38) and PKC(s). CaN phosphatase directly binds to nSMase2, dephosphorylates it, and thereby, deactivates it, as shown previously by us (21). ox-stress causes CaN degradation, leading to nSMase2 phosphorylation. We identified five serines phosphorylated in nSMase2 (see also Fig. 1). Phosphorylation of serine 173 is necessary to impose the activation of nSMase2 following ox-stress exposure of HAE cells, whereas the phosphorylation of serine 208 may be involved in increasing nSMase2 protein stability while interdependent regulation between phosphorylation sites occurs. Overall, increased phosphorylation of nSMase2 leads to its activation and its augmented protein stability, and as a result of these steps, ceramide levels are elevated by sphingomyelin hydrolysis.