Figure 3.
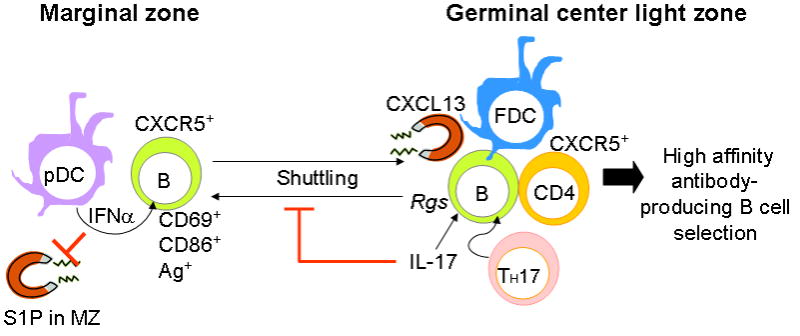
Migration of marginal zone precursor B cells regulated by type I interferon (IFN) and IL-17. This figure depicts our current view on the migration and migration arrest of CD86hi antigen-bearing marginal zone precursor B cells. Our results suggest that type I IFN produced by plasmacytoid dendritic cells (pDCs) in the marginal zone (MZ) provides a signal to upregulate CD69. This downregulates S1P1 and the S1P chemotactic response to favor the CXCL13-induced follicular entry of marginal zone precursor B cells. A second action in the germinal center (GC) results from production of CXCL13 by follicular dendritic cells (FDCs). CXCR5 is highly expressed on marginal zone precursors and CXCL13 from FDCs can attract these B cells into an active developing GC. A third action comes from IL-17 produced by T helper 17 cells (TH17) that were in the GC region. IL-17 signals through the NF-κB pathway in B cells to upregulate RGS. This locks the marginal zone precursor B cells into their position in the GC FDC region to interact with effector CXCR5+ CD4 T cells. Thus, the formation of spontaneous GCs in the spleens of BXD2 mice is highly associated with localized imbalance of cytokines that are normally invoked during immune responses. Such coupled effects of cytokines on different cells at different anatomic locations within a lymphoid organ may result in responses that are not predicted by the analysis of the effects the isolated cytokines on stationary cells (Ag: antigen; FDC: follicular dendritic cells; MZ: marginal zone; pDC: plasmacytoid dendritic cells; Rgs: regulators of G-protein signaling; S1P: sphingosine-1- phosphate; TH-17: T helper 17 cells)
