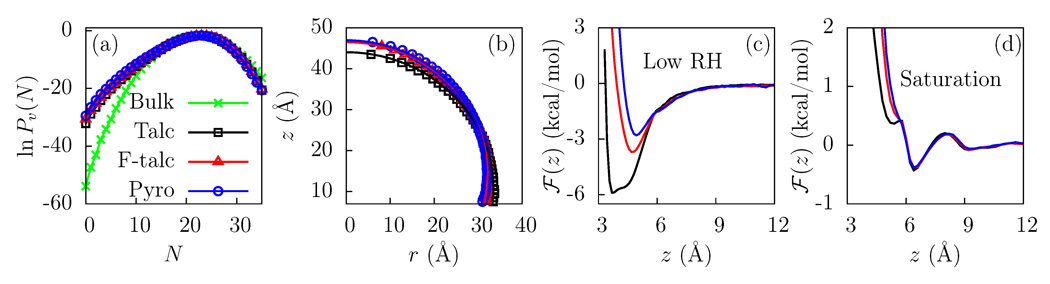
Figure 2.
(a) The probability, Pv(N), of observing N water molecules in a probe volume (v = 15 × 15 × 3 Å3) displays a low N fat tail when v is near the surface of talc (black), fluorotalc (red), and pyrophyllite (blue), as compared to that when v is in bulk water (green). (b) Water droplet profiles corresponding to ρ(r, z) = 0.5ρb are shown for the clay surfaces. The contact angles for the surfaces are similar: 96° for talc, 103° for fluorotalc, and 105° for pyrophyllite (based on tangents drawn at zS = 7Å). (c) Potential of mean force, ℱ(z), for the adsorption of an isolated water molecule (low RH) to the clay surfaces. The hydrogen atoms of the talc −OH groups are located at z = 2 Å and can participate in hydrogen bonds with water molecules. (d) ℱ(z) at the clay - liquid water interface (saturation). To maximize H-bonding with other waters, the binding site is no longer occupied.