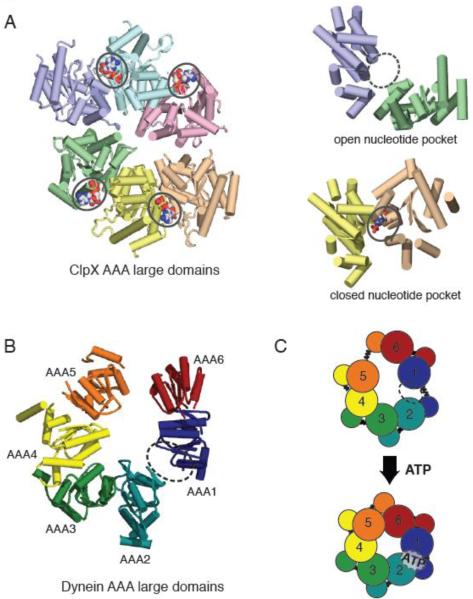
Figure 3.
(A) X-ray crystal structure of the large domains of the ClpX hexamer (PDB ID: 3HWS), color coded by chain. The nucleotide-free domain interfaces (purple-green) show an open conformation, while the nucleotide-bound (ADP) domain interfaces (yellow-peach) show a closed conformation. (B) Asymmetric structure of the yeast cytoplasmic dynein AAA ring (only large domains shown). When viewed from the linker-face, the ring shows prominent openings between AAA1-AAA2 and AAA5-AAA6 large domains. The AAA1-AAA2 interface is the main ATP hydrolysis site for dynein. (C) A model for AAA ring communication in dynein, based upon the nucleotide-free and bound forms of ClpX. Upon ATP binding at the AAA1 nucleotide binding pocket, the AAA1-AAA2 switches from an open to closed conformation, triggering an overall shift in AAA domains, and ultimately an iris-like contraction of the AAA ring.