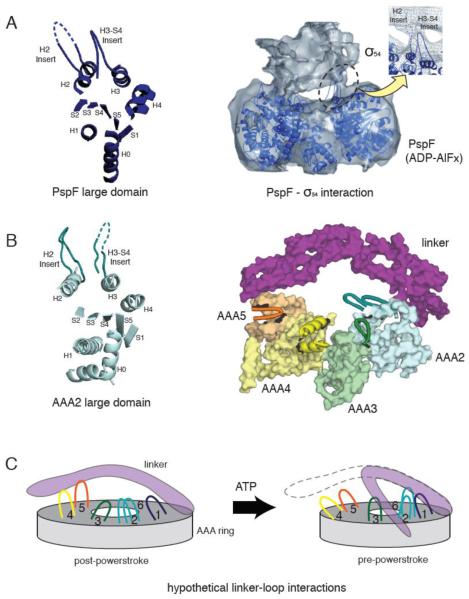
Figure 4.
(A) The crystal structure of a PspF large domain showing the position of the H2 and H3-S4 insert loops (left). A 20 Å resolution cryo-electron microscopy reconstruction of the PspF hexamer in complex with its binding partner, σ54 in the presence of ADP-AlFx (right). The PspF hexamer crystal structure is docked into the electron microscopy structure, showing the H2 and H3-S4 insert loops at the site of PspF - σ54 interaction (reprinted from [46] with permission). (B) The crystal structure of AAA2 large domain in yeast cytoplasmic dynein, showing a similar position of the H2 and H3-S4 insert loops compared with PspF (left). The position of the unique loop inserts in the large domains of dynein AAA2-5, with respect to the linker (right). AAA1 and AAA6 are not shown for clarity. Insert loops lying at the top surface of the ring are depicted in cartoon format. The loops provide potential docking sites for the linker or dynein regulatory proteins. (C) A speculative model of how the loop insertions could act as docking sites for the linker at different stages of the ATPase cycle.