Abstract
Several Angiopoietin-like (ANGPTL) molecules have been implicated in enhancement of ex-vivo expansion of murine and human (hu) hematopoietic stem cells, but there are no reports on hematopoietic progenitor cells (HPCs). We assessed purified recombinant endotoxin-free hu ANGPTL-2 Coiled-Coil (CC), -3, -3CC, -3 fibrinogen-like domain (FLD), -4, -4CC, -5CC, -6 and -7 for effects on proliferation and survival of HPCs from hu cord blood (CB). None of the ANGPTL molecules stimulated CB HPC proliferation, or enhanced or inhibited colony formation of CB HPC stimulated by various growth factors. However, ANGPTL-2CC, -3, and -3CC significantly enhanced survival of HPC (CFU-GM, BFU-E, CFU-GEMM) subjected to delayed addition of growth factors. Survival enhancing effects of ANGPTL-3 were neutralized by purified anti-ANGPTL-3, but not by anti-ANGPTL-4, -6, or -7. ANGPTL-2CC, -3, and -3CC, but not -4, -6, or -7 also enhanced replating capacity of single CB CFU-GEMM colonies, an estimate of the self-renewal capabilities of HPCs, by greater than 2 fold. Effects of at least ANGPTL-3CC may in part be mediated through phosphorylation of ERK. The ANGPTL molecules did not influence ex-vivo expansion of hu CB CD34+ cells, alone, or in combination with SCF, TPO, Flt3-ligand, with or without IL-3. Thus, amongst ANGPTL family members, ANGPTL-2 and -3 had enhancing activities on human HPC survival and replating activity, effects requiring the CC domain of the ANGPTL molecules. This information is of relevance to hu HPC regulation.
Keywords: Hematopoietic Progenitor Cells, Cord Blood, Cell Survival, Angiopoietin-Like Proteins
Introduction
Cytokines, chemokines and growth factors regulate HSC and HPC proliferation, self-renewal, survival, and differentiation [1]. Several members of the Angiopoietin-like (ANGPTL) family of secreted proteins, which have characteristic structures, but not similar activities, of angiopoietins, have been implicated in enhancement of cytokine-dependent ex-vivo expansion of murine fetal liver and bone marrow HSCs, and hu CB NOD/SCID-repopulating cells (SRCs) [2–5]. ANGPTL-2, -3, -5, and -7 enhanced ex-vivo expansion of long-term repopulating mu HSCs in the presence of saturating amounts of other cytokines [2]. ANGPTL-3 null mice were suboptimal recipients for engraftment of normal murne (mu) bone marrow cells, demonstrating the need for endogenous ANGPTL-3 for optimal engrafting capability of HSCs [3]. ANGPTL-5 enhanced cytokine-dependent expansion of hu CB CD34+ SRCs [4], and mesenchymal stromal cells, genetically-engineered to express ANGPTL-5 supported expansion of hu CB SRCs [5]. To date, there have not been any reports on possible activities of ANGPTL molecules on HPC function. HPCs are an important population of immature hematopoietic cells, that link HSCs and mature blood cells in a catenated hierarchy of blood cell production [1]. Here, we evaluated purified recombinant (r) hu ANGPTL-2, -3, -4, -5, -6, and -7 for effects on proliferation, survival, and replating capacity of hu CB HPCs. ANGPTL molecules include a single peptide, an extended helical domain predicted to form dimeric or trimeric coiled-coils (CC), a short linker peptide, and a globular fibrinogen-like domain (FLD). We found that ANGPTL-2, and -3 enhanced survival of hu CB HPCs subjected to delayed addition of cytokines, and the replating capacity of hu CB HPCs, both effects requiring the CC domain ANGPTL-2, and -3, without effects on proliferation or ex-vivo expansion of HPCs.
Materials and Methods
Reagents
Purified endotoxin-free (<0.1 Eu/ug endotoxin per LAL method) rhu ANGPTL-2 CC, -3, -3CC, -3 FLD, -4, -4CC, -5CC, -6, and -7, and purified rabbit hu ANGPTL-3, -4, -6, and -7 IgG, were from Adipogen, Inc. (Incheon, Korea). Purified rhu Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF), Interleukin-3 (IL-3), Stem Cell Factor (SCF), Flt3-ligand (FL), and Stromal Cell Derived Factor-1 (SDF-1/CXCL12) were purchased from R&D Systems (Minneapolis, MN). Purified rhu Erythropoietin (EPO) was purchased from Amgen, Inc. (Thousand Oaks, South San Francisco, CA). Phospo-specific and total Erk antibodies were purchased from Cell Signaling Technology, Inc. ( Danvers, MA) for western blot analysis.
Cells and HPC Assays
Hu CB cells were obtained from the Wishard Hospital, Indiana University (IU) School of Medicine, and approved for use by the IU IRB. CB was separated by density cut procedure into a low density (LD, <1.077 gm/cm3) fraction by Ficol-Hypaque, or further isolated into a >95% pure CD34+ cell population by magnetic bead separation as previously reported [6].
Colony Assays for HPC (granulocyte-macrophage (CFU-GM, Colony Forming Unit-Granulocyte Macrophage), erythroid (BFU-E, Burst Forming Unit-Erythroid), and multipotential (CFU-GEMM, CFU-granulocyte, erythroid, macrophage, megakaryocyte) proliferation [6], survival [7,8], replating [9–11], and ex-vivo expansion [12] were done as reported.
Statistics
For assays, a 2-tailed students t test was used with significant changes at least p<0.05.
Results
There are a number of cytokines that can stimulate, enhance or suppress colony formation by HPCs [1]. As there were no reports of ANGPTL molecules on HPC effects, we tested ANGPTL-2CC, -3, -3CC, -3FLD, -4, -4CC, -5CC, -6, and -7 at concentrations of 50, 100, 250, and 500 ng/ml for their capacity to stimulate colony formation by CFU-GM, BFU-E and CFU-GEMM in LD CB cells (plated at 2.5 and 5.0 × 104 cells/ml) in the absence of any added cytokines (no colonies formed in control). We also tested these same concentrations of ANGPTL molecules to see if they enhanced or suppressed: CFU-GM colony formation stimulated by GM-CSF (10nl/ml) (control colonies >20), GM-CSF (10ng/ml) plus SCF (50ng/ml) (control colonies >60), EPO (1U/ml) and SCF (50ng/ml) (control colonies >5), or Epo (1U/ml) plus SCF (50ng/ml), GM-CSF (10ng/ml) and IL-3 (10ng/ml) (control colonies >40), BFU-E colony formation stimulated by Epo (1U/ml) plus SCF (50ng/ml) (control colonies >12), and/or CFU-GEMM colonies stimulated by Epo (1U/ml) plus SCF (50ng/ml), or Epo (1U/ml) plus SCF (50ng/ml), GM-CSF (10ng/ml), and IL-3 (10ng/ml) (control colonies >80). In two complete experiments, none of the ANGPTL molecules at any of the tested concentrations stimulated or significantly altered cytokine-stimulated colony formation by CFU-GM, BFU-E, or CFU-GEMM.
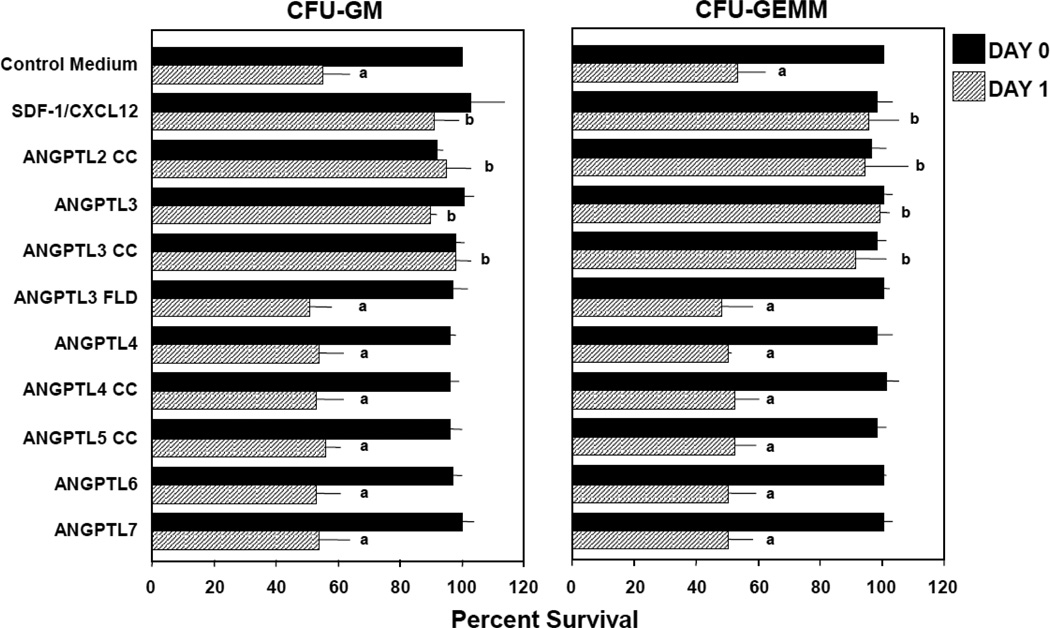
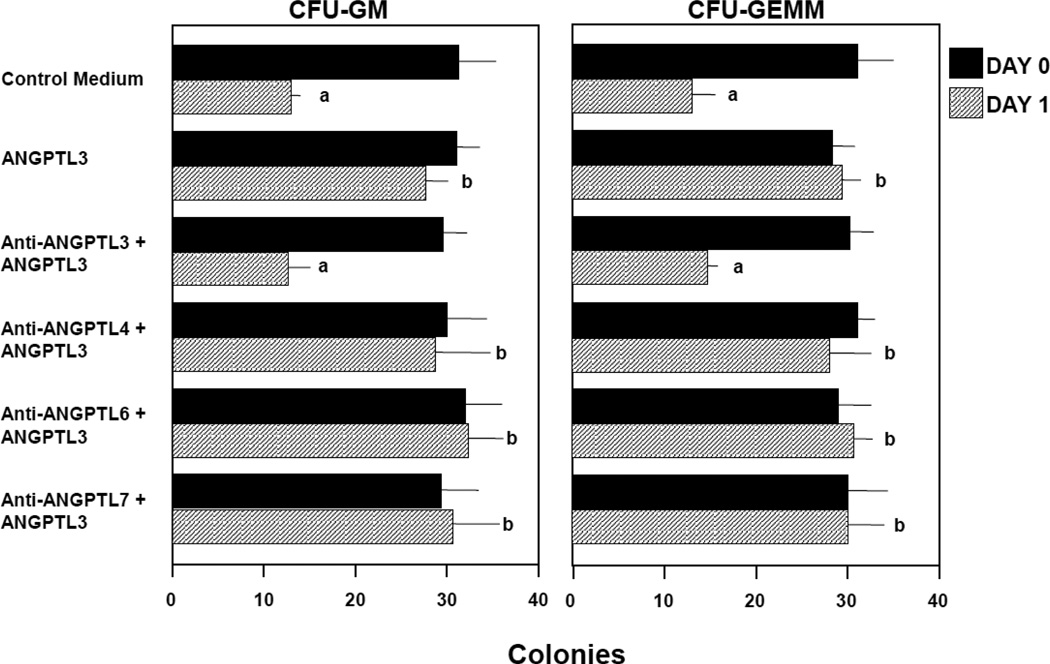
Since there are cytokines (such as SDF-1/CXCL12) (1) that neither stimulate, enhance nor suppress proliferation of HPC, but which enhance the survival of HPC in context of delayed addition of cytokines, a means to measure apoptosis [7–9], we compared all the above mentioned ANGPTL molecules, at concentrations of 10, 100, 200 and 500ng/ml, for their capacity to enhance colony formation by CFU-GM and CFU-GEMM in 2.5 × 104 LD, and 500 CD34+, CB cells, subjected to 24–48 hr delayed addition of Epo (1U/ml), SCF (50ng/ml), GM-CSF (10ng/ml) and IL-3 (10ng/ml) in comparison to that of 100ng/ml SDF-1/CXCL12 a known HPC survival enhancing molecule [7–9]. The average results (mean ±1SD) for 4–5 experiments of effects on LD cells, including 2 experiments on CD34+ cells (shown in Figure 1 for 24 hr delayed cytokine addition) for CFU-GM and CFU-GEMM, demonstrate that 200ng/ml ANGPTL-2CC, -3, and -3CC, but not up to 500ng/ml ANGPTL-3 FLD, -4, -4CC, -5CC, -6, or -7, significantly enhanced the survival of CFU-GM and CFU-GEMM. Not shown is that 100ng/ml, but not 10ng/ml, of ANGPTL-2CC, -3, and -3CC was effective in significantly enhancing survival of CFU-GM or CFU-GEMM and that 500ng/ml ANGPTL-2CC, -3, and -3CC was as effective as 200ng/ml of these molecules, thus demonstrating a dose-dependent effect for ANGPTL-2 and -3, and highlighting the importance of the CC domain of these molecules for this activity. Since ANGPTL 3 FLD was not active, the FLD domain does not appear to be necessary for this activity, at least for ANGPTL-3. As shown in Figure 2, the survival enhancing effect of ANGPTL-3 was neutralized by purified rabbit anti ANGPTL-3 IgG, but not by anti-ANGPLT-4, -6, or -7 IgG, demonstrating the specificity of ANGPTL-3 in this effect, both by use of a purified rhu ANGPTL-3 molecule, and its specific neutralization with anti ANGPTL-3. Replating of CFU-GEMM-colonies offers an estimate of the limited “self-renewal” capacity of CFU-GEMM [10,11]. In this context, we found that ANGPTL-2CC, -3, and -3CC, but not -3FLD, -4, -4CC, -6, or -7 significantly enhanced the percent secondary colonies per replated primary CFU-GEMM colony derived from 2.5 × 104 LD or 500 CD34+ CB cells/ml (Table 1). SDF-1/CXCL12, also known to enhance replating of CFU-GEMM colonies [11], was used as a positive control for replating efficiency. However, this enhancement of replating effectiveness of ANGPTL-2CC, -3, and -3CC did not translate into enhancement of cytokine (SCF (50ng/ml) TPO (10ng/ml), and FL (100ng/ml))-dependent ex-vivo expansion of CB HPC, as neither ANGPTL-2CC, -3, -3CC, -3FLD, -4, -4CC, 5CC, -6, nor -7 significantly enhanced ex-vivo expansion, in contrast to SDF-1/CXCL12, which did enhance such cytokine-dependent ex-vivo expansion (Table 2). Ex-vivo cultures in Table 2 were initiated with 1000 CD34+ CB cells with and without the above noted cytokine cocktail for 7 days, and with and without 500ng/ml ANGPTL molecules, with all molecules placed with the cells at time zero. None of the ANGPTL-molecules by themselves allowed ex-vivo expansion in the absence of SCF, TPO and FL (data not shown), nor did the ANGPTL molecules influence cytokine induced enhancement of HPC total nucleated cells, CD34+ cell counts or cell cycle status of cultured cells at different times (data not shown).
Figure 1.
Influence of angiopoietin-like (ANGPTL) proteins on survival of human cord blood granulocyte macrophage (CFU-GM) and multipotential (CFU-GEMM) progenitor cells subjected to delayed addition of growth factors (GFs). Results are shown as mean +/− 1SD for 4–5 experiments each, with 100ng/ml SDF-1/CXCL12, 200ng/ml of either ANGPTL-2CC, -3, or -3CC, and 500ng/ml of either ANGPTL-3FLD, -4, -4CC, -5CC, -6 or -7. GFs used were 1U/ml rhu EPO, 10ng/ml rhu GM-CSF and rhu IL-3, and 50ng/ml rhu SCF. Colonies were scored 14 days after addition of GFs. asignificantly different from day 0 control medium (p<l0.001); bsignificantly different from day 1 control medium counts (p<0.001).
Figure 2.
Anti-ANGPTL-3, but not anti-ANGPTL-4, -6, or -7 neutralizes the survival enhancing effects of ANGPTL-3. Results are shown as the mean +/− 1SD for on experiment in which 500 CD34+ cord blood cells were plated per ml. ap<0.001 compared to day 0 control medium; b p<0.001 compared to day 1 control medium.
Table 1.
Angiopoietin-Like (ANGPTL) Proteins 3, 2CC, and 3CC, but not 3FLD, 4, 4CC, 6 and 7, Enhance Replating Capacity of Human Cord Blood Multipotential Progenitor Cells.
| A) | # Colonies formed in 2° plate/#CFU-GEMM colonies replated | # secondary colonies per replated primary colony | |
|---|---|---|---|
| Control Medium | 428 / 54 | 7.9 | |
| SDF-1/CXCL12 | 1058/34 | 31.1* | |
| ANGPTL 3 | 976/36 | 27.1* | |
| ANGPTL 3CC | 811 /56 | 14.5* | |
| ANGPTL 3FLD | 457 / 51 | 9.0 | |
| ANGPTL 4 | 374 / 49 | 7.6 | |
| ANGPTL 4CC | 467 / 50 | 9.3 | |
| ANGPTL 6 | 596 / 53 | 11.2 | |
| ANGPTL 7 | 547 / 51 | 10.7 | |
| B) | # Colonies formed in 2° plate/#CFU-GEMM colonies replated | # secondary colonies per replated primary colony | |
| Control Medium | 27/12 | 1.3 | |
| SDF-1/CXCL12 | 93/19 | 4.9* | |
| ANGPTL2CC | 82/20 | 4.1* | |
| ANGPTL3 | 212/21 | 10.1* | |
| ANGPTL3CC | 254 / 21 | 12.1* |
p<0.01 compared to control medium
Table 2.
Angiopoietin-Like (ANGPTL) Proteins Do Not Enhance Ex-Vivo Expansion of Hematopoietic Progenitor Cells Stimulated by the Combination of SCF, TPO, and Flt3-Ligand
| Test Material | Ex-Vivo Expansion (Fold Increase) | |||||
|---|---|---|---|---|---|---|
| CFU-GM | BFU-E + CFU-GEMM |
|||||
| Exp#1 | Exp#2 | Exp#3 | Exp#1 | Exp#2 | Exp#3 | |
| Control Medium | 10.2 | 9.1 | 15.5 | 2.3 | 1.5 | 4.0 |
| SDF-1/Diprotin A | ND | ND | 39.5* | ND | ND | 6.3* |
| ANGPTL2CC | ND | ND | 16.0 | ND | ND | 3.2 |
| ANGPTL3 | 10.8 | 8.7 | 18.6 | 2.9 | 1.7 | 3.4 |
| ANGPTL3CC | 9.7 | 8.5 | 16.5 | 2.5 | 1.5 | 3.7 |
| ANGPTL3FLD | 8.5 | 8.9 | 16.0 | 1.9 | 1.7 | 3.7 |
| ANGPTL4 | 11.2 | 8.5 | 17.0 | 2.7 | 1.7 | 3.2 |
| ANGPTL4CC | 10.6 | 8.7 | 16.5 | 2.7 | 1.7 | 3.2 |
| ANGPTL5CC | ND | ND | 16.9 | ND | ND | 3.4 |
| ANGPTL6 | 11.5 | 8.9 | 16.0 | 2.1 | 1.7 | 3.2 |
| ANGPTL 7 | 9.0 | 9.3 | 16.5 | 2.2 | 1.7 | 3.7 |
Significant increase, p< 0.01 compared to control medium. ND, experimental point not set up.
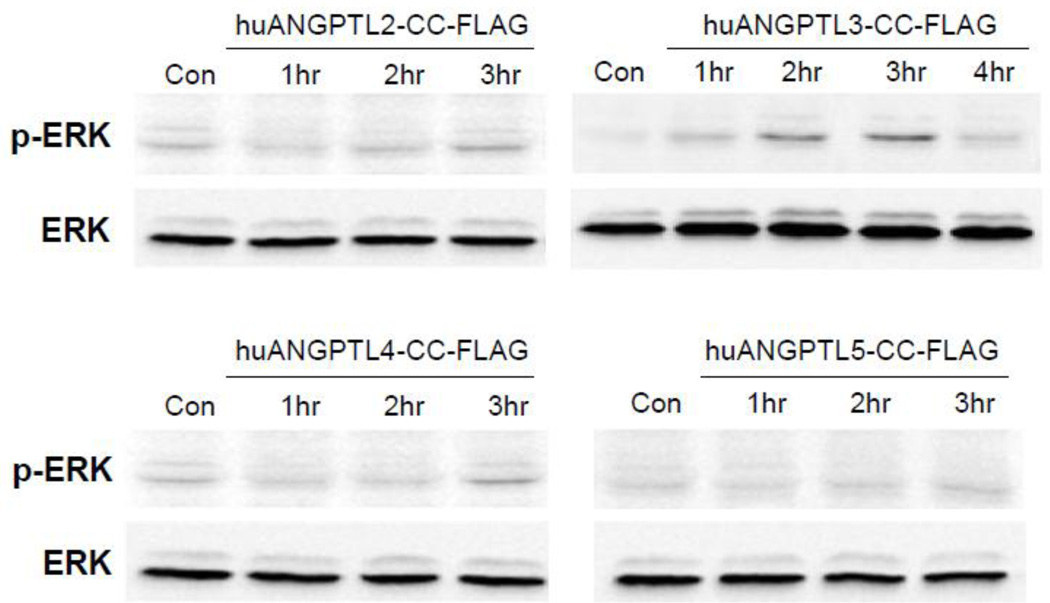
While the FLD of some ANGPTLs are known to be ligands for some intergrins [13,14] the receptor(s) for CC domains of the ANGPTL family of molecules remain(s) largely unknown. To see if CC domains of some ANGPTLs are able to elicit cell signaling, THP.1 cells, a human monocytic leukemia cell line, were stimulated with the CC domain of ANGPTL-2, -3, -4, or -5, and phosphorylation of Erk was examined. Only the CC domain of ANGPTL3 caused sustained phosphorylation of Erk in THP.1 cells as shown in Figure 3.
Figure 3.
Phosphorylation of Erk by the CC domain, depicted as CC-FLAG, of human ANGPTL-3. Recombinant CC domains were expressed as FLAG-tagged form in HEK293 cells and purified. Human monocytic leukemic cell line, THP.1 cells were left serum-starved overnight and stimulated with 500ng/ml each for CC domains of ANGPTL-2, -3, -4, and -5 for the indicated time points. Cell lysates were then prepared and subjected to western blot.
Discussion
Our results present a here-to-fore unknown function for ANGPTL -2, and -3. Of interest is the requirement of the CC domain of these two ANGPTL-molecules for their capacity to enhance the survival of CB CFU-GM and CFU-GEMM subjected to delayed addition of cytokines, a procedure known to cause apoptotic death of HPCs [1,7,8], and for enhancing the replating capacity of CFU-GEMM, a measure of the estimated limited self-renewal capacity of CFUGEMM [10–12]. However, none of the ANGPTL molecules without other cytokines expanded CB HPCs, or enhanced cytokine-dependent ex-vivo expansion of hu CB HPCs. This is of interest in context of studies demonstrating that ANGPTL-2, -3, -5, and -7 do enhance cytokine stimulated ex-vivo expansion of mouse HSCs, and that ANGPTL-5 enhances cytokine stimulated ex-vivo expansion of hu CB SRCs. HSCs/SRCs are earlier, more immature cells than HPCs in the hierarchy of blood cell production [1]. It would seem that the ANGPTL ex-vivo enhancing effects of HSC, do not apply to such effects on HPC, unless, the ex-vivo effects on HPC in our system were blocked or counteracted by serum used in our system. Serum was not used in the ex-vivo expansion studies by others [2–4]. We do not feel that serum in our system played a role in our negative results for ex-vivo expansion, as serum was present in the survival and replating studies and positive effects of ANGPTL-2, and -3 were apparent. Regardless, the cell survival effects we noted for ANGPTL-2, and -3 on hu CB HPC may complement the effects noted by others on mu HSCs/hu SRCs as cell survival may in part play a role in their studies. Information on actions of ANGPTL molecules is still very limited. There has been a focus on the metabolic roles of some members of the ANGPTL molecules, with ANGPTL-3 and -4, the closest related molecules in terms of structure and metabolic function being potent lipoprotein lipase and endothelial lipase inhibitors, where they may play a role in keeping blood low density and high density lipid, and triglyceride, levels constant [13]. There are also emerging roles for ANGPTL-2 and -6 in lipid metabolism [15]. The CC domains of ANGPTL-3 and -4 are responsible for the above lipase inhibitory function [16], whereas the requirements of the CC domain of ANGPTL-2 and -6 for these activities is not known. Due to amino acid sequence divergence of the CC domains between ANGPTL-3/ANGPTL-4 and ANGPTL-2/ANGPTL-6 it does seem not likely that the CC domain of ANGPTL-2 or ANGPTL-6 can function as a molecular inhibitor of the lipases. Whether the ANGPTL-2 and/or -3 activities we noted on survival/replating of hu HPC relate to effects on lipid metabolism is not known, although it may be telling in terms of distinguishing such effects of ANGPTL-2, -3, -4, and -6. In our assays, only ANGPTL-2 and -3 were active. Interestingly, we demonstrated that only the CC domain of ANGPTL-3 enhanced phosphorylation of Erk. Due to the respective similarity and disparity in terms of amino acid sequence of the CC domain of ANGPTL-3 with that of ANGPTL-4 and ANGPTL2, we speculate that the receptor for the CC domain of ANGPTL-3 that exerts its survival/replating of hu HPC, may be unique, a possibility worth future investigation. Exactly how these molecules work at a receptor and intracellular level, and in what specific context, requires further investigation. Some evidence for repression of the expression of the transcription factor I karos by ANGPTL3 has been reported [3], but until the receptors for the different ANGPTL molecules are identified and characterized, we will not likely understand exactly how the ANGPTL molecules manifest their receptor-mediated effects within the target cells, or for that matter exactly what the target cells (e.g. HSCs, HPCs, or accessory cells that then influence HSCs/HPCs) are. To demonstrate that ANGPTL molecules exert their effects directly on HSCs or HPCs, two requirements must be met. First, assays should be conducted on extremely purified populations of these cells. None of the studies reported to date have used cell populations with such a high degree of purity. Second, studies would have to be done at the single isolated cell level using highly purified individual HSCs or HPCs as previously reported for other cytokines [7,17,18].
Acknowledgements
These studies were supported by US Public Health Service Grants R01 HL67384 and R01 HL56416 from the NIH to HEB, and R01 HL55716 to EFS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shaheen M, Broxmeyer HE. The humoral regulation of hematopoiesis. In: Hoffman R, Benz EJ Jr, Shattil SJ, Furie B, Silberstein LE, McGlave P, Heslop H, Anastasi J, editors. Hematology: Basic Principles and Practice, Chapter 24. 5th Edition. Philadelphia, PA: Elsevier Churchill Livingston; 2009. pp. 253–275. Part III. [Google Scholar]
- 2.Zhang CC, Kaba M, Ge G, et al. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat.Med. 2006;12:240–245. doi: 10.1038/nm1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng J, Huynh H, Umikawa M, et al. Angiopoietin-like protein 3 supports the activity of hematopoietic stem cells in the bone marrow niche. Blood. 2011;117:470–479. doi: 10.1182/blood-2010-06-291716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang CC, Kaba M, Iizuka S, et al. Angiopoietin-like 5 and IGFBP2 stimulate ex vivo expansion of human cord blood hematopoietic stem cells as assayed by NOD/SCID transplantation. Blood. 2008;111:3415–3423. doi: 10.1182/blood-2007-11-122119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khoury M, Drake A, Chen Q, et al. Mesenchymal stem cells secreting angiopoietin-like-5 support efficient expansion of human hematopoietic stem cells without compromising their repopulating potential. Stem Cells Dev. 2011;20:1371–1381. doi: 10.1089/scd.2010.0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J.Exp.Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broxmeyer HE, Kohli L, Kim CH, et al. Stromal cell-derived factor-1/CXCL12 directly enhances survival/antiapoptosis of myeloid progenitor cells through CXCR4 and G(alpha)i proteins and enhances engraftment of competitive, repopulating stem cells. J.Leukoc.Biol. 2003;73:630–638. doi: 10.1189/jlb.1002495. [DOI] [PubMed] [Google Scholar]
- 8.Broxmeyer HE, Cooper S, Kohli L, et al. Transgenic expression of stromal cell-derived factor-1/CXC chemokine ligand 12 enhances myeloid progenitor cell survival/antiapoptosis in vitro in response to growth factor withdrawal and enhances myelopoiesis in vivo. J.Immunol. 2003;170:421–429. doi: 10.4049/jimmunol.170.1.421. [DOI] [PubMed] [Google Scholar]
- 9.Carow CE, Hangoc G, Cooper SH, et al. Mast cell growth factor (c-kit ligand) supports the growth of human multipotential progenitor cells with a high replating potential. Blood. 1991;78:2216–2221. [PubMed] [Google Scholar]
- 10.Carow CE, Hangoc G, Broxmeyer HE. Human multipotential progenitor cells (CFU-GEMM) have extensive replating capacity for secondary CFU-GEMM: an effect enhanced by cord blood plasma. Blood. 1993;81:942–949. [PubMed] [Google Scholar]
- 11.Broxmeyer HE, Mejia JA, Hangoc G, et al. SDF-1/CXCL12 enhances in vitro replating capacity of murine and human multipotential and macrophage progenitor cells. Stem Cells Dev. 2007;16:589–596. doi: 10.1089/scd.2007.0044. [DOI] [PubMed] [Google Scholar]
- 12.Broxmeyer HE, Srour EF, Hangoc G, et al. High-efficiency recovery of functional hematopoietic progenitor and stem cells from human cord blood cryopreserved for 15 years. Proc.Natl.Acad.Sci.U.S.A. 2003;100:645–650. doi: 10.1073/pnas.0237086100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camenisch G, Pisabarro MT, Sherman D, et al. ANGPTL3 stimulates endothelial cell adhesion and migration via integrin alpha vbeta 3 and induces blood vessel formation in vivo. J.Biol.Chem. 2002;277:17281–17290. doi: 10.1074/jbc.M109768200. [DOI] [PubMed] [Google Scholar]
- 14.Goh YY, Pal M, Chong HC, et al. Angiopoietin-like 4 interacts with integrins beta1 and beta5 to modulate keratinocyte migration. Am.J.Pathol. 2010;177:2791–2803. doi: 10.2353/ajpath.2010.100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadomatsu T, Tabata M, Oike Y. Angiopoietin-like proteins: emerging targets for treatment of obesity and related metabolic diseases. FEBS J. 2011;278:559–564. doi: 10.1111/j.1742-4658.2010.07979.x. [DOI] [PubMed] [Google Scholar]
- 16.Yau MH, Wang Y, Lam KS, et al. A highly conserved motif within the NH2-terminal coiled-coil domain of angiopoietin-like protein 4 confers its inhibitory effects on lipoprotein lipase by disrupting the enzyme dimerization. J.Biol.Chem. 2009;284:11942–11952. doi: 10.1074/jbc.M809802200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinzfogl J, Hangoc G, Broxmeyer HE. Neurexophilin 1 suppresses the proliferation of hematopoietic progenitor cells. Blood. 2011;118:565–575. doi: 10.1182/blood-2010-12-325381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srour EF, Tong X, Sung KW, et al. Modulation of in vitro proliferation kinetics and primitive hematopoietic potential of individual human CD34+CD38−/lo cells in G0. Blood. 2005;105:3109–3116. doi: 10.1182/blood-2004-05-1773. [DOI] [PubMed] [Google Scholar]