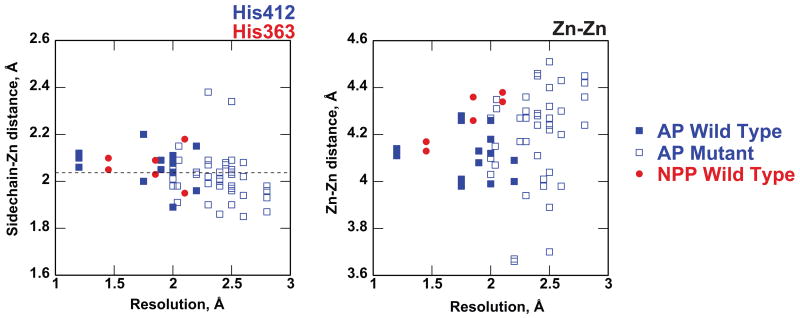
Figure 5.
Comparison of Zn-sidechain and Zn-Zn distances among the available structures of AP (blue) and NPP (red). Zinc contacts with His 412 (AP) and His363 (NPP) are given as an example, and comparisons for all other zinc-ligand interactions are provided in the Supplemental Material. Wild-type AP structures are shown in closed squares and mutants are shown in open squares. Both free enzymes and enzymes bound to substrate and transition state analogs are included in the plots. Each point represents a single active site within the asymmetric unit of the corresponding crystal structure. The dashed line indicates average interaction distances from zinc-nitrogen interactions from small-molecule crystal structures 63,66.