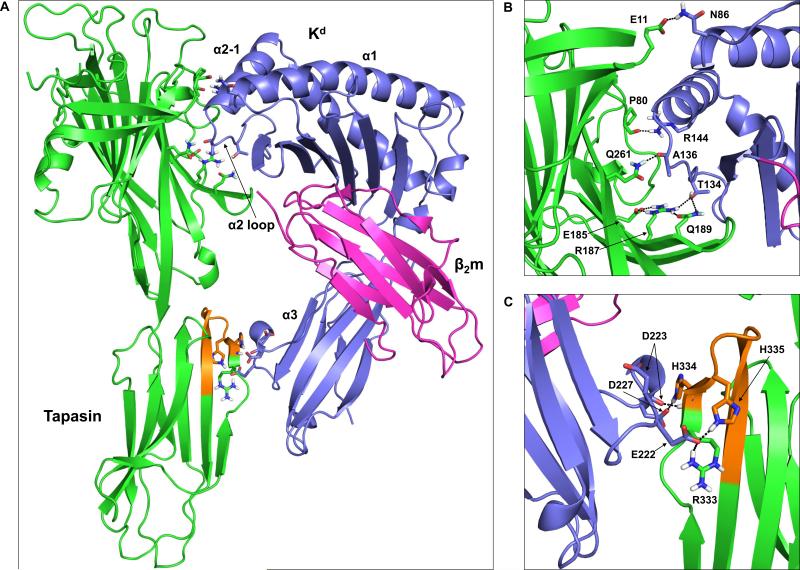
Figure 6. Molecular model of the tapasin-Kd interaction reveals possible intermolecular interactions involving the tapasin N-terminal Ig-like domain, as well as tapasin residues within the 334-342 loop.
(A) The tapasin crystal structure (PDB code 3F8U) (Dong et al., 2009) was docked with the open, peptide-free form of Kd (generated from the crystal structure of peptide-bound Kd, PDB code 2FWO (Mitaksov and Fremont, 2006)) as described in the Materials and methods. Tapasin is shown in green with residues 334-342 in orange. The Kd heavy chain is shown in purple, and β2m is in magenta. Also labeled on the Kd structure are the α1 helix, the α2 domain loop, the short α2-1 helix, and the α3 domain. (B) Interactions involving residues within the MHC class I α1/α2 domains and the tapasin N-terminal Ig-like domain. The amino acids that are involved in hydrogen bonds/salt bridges (see also Table I) are shown as sticks and labeled. In addition to these intermolecular interactions, an intramolecular hydrogen bond between tapasin R187 and Q189, and an intramolecular salt bridge between tapasin R187 and E185 are shown. (C) Interactions involving residues within the MHC class I α3 domain and the tapasin C-terminal Ig-like domain are indicated. The orientation shown in (C) is rotated 180° from that shown in (A).