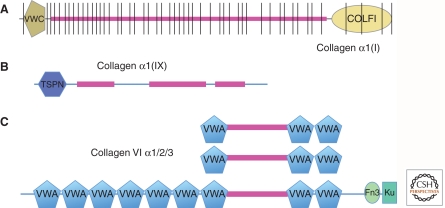
Figure 1.
Examples of collagen structures. (A) Collagen I is a fibrillar collagen with a continuous collagen domain of around 1000 amino acids (fuschia) comprising Gly-X-Y repeats that form a triple helix. It is encoded by multiple exons (note vertical lines) that are variants of a primordial exon encoding six such repeats. The collagen domain is flanked by amino- and carboxy-terminal noncollagenous domains that are removed by proteolysis to allow fibrillogenesis of the mature collagen. The VWC domain in this and other fibrillar collagens can be alternatively spliced and binds bone morphogenetic proteins (BMPs). (B) Collagen IX is a FACIT collagen (fibril-associated collagen with interrupted triple helix); the interruptions in the collagen domain allow bending. This and other FACIT collagens associate with fibrillar collagens and their amino-terminal domains extend out from the fibrils and presumably function as protein-binding domains. (C) Collagen VI is a heterotrimer of three related subunits, one of which is much longer and forms globular heads at each end. VWA domains are commonly protein-binding domains and probably allow interactions with other proteins during the formation of short fibrils by collagen VI.